Synthesis and Spectral Studies on Substituted Metal (II) -Tetra-1-(Thiophene-2-yl)methaniminephthalocyanine Complexes
Department of Chemistry, Manipal Institute of Technology, MAHE, Manipal, Udupi District, Karnataka, India.
Corresponding Author E-mail: fasiulla1976@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340344
Article Received on : March 08, 2018
Article Accepted on : April 23, 2018
Article Published : 21 Jun 2018
The synthesis, characterization of symmetrically substituted transition metal (ΙΙ) – tetra-1-(thiophene-2-yl) methaniminephthalocyanine complexes by condensation of tetraamino phthalocyanines with 2- thiophenecarboxaldehyde had been prepared. The structural and characterization of theblue colour tetra -1- (thiophene-2- yl) methaniminephthalocyanine complexesare elucidated by using a number of analytical techniques like FT -Infrared , UV-Vis spectroscopy, XRD, magnetic measurements and thermo-gravimetric analysis.The kinetic parameters and thermal decomposition of synthesized phthalocyanines complexes were calculated using thermo-gravimetric analytical data.
KEYWORDS:IR Spectra; Magnetic Measurements; Powder Diffraction; Tetra-(Thiophene-2-Yl) Methanimine; X-Ray TGA
Download this article as:| Copy the following to cite this article: Ulla F, Yashoda M. P. Synthesis and Spectral Studies on Substituted Metal (II) -Tetra-1-(Thiophene-2-yl)methaniminephthalocyanine Complexes. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Ulla F, Yashoda M. P. Synthesis and Spectral Studies on Substituted Metal (II) -Tetra-1-(Thiophene-2-yl)methaniminephthalocyanine Complexes. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=46806 |
Introduction
As the metal phthalocyanines were prominent as dyes and pigments, so the first and foremost interest was taken in them. Phthalocyanines are commercial blue-green coloured pigments too.The blue-green colour is the result of strong absorption in the Q-band, the visible region of the spectrum. The succeeded intensive research is aimed at proving into, how the produce of the phthalocyanines was used sensitizers in photodynamic therapy and other medical applications and phthalocyanine derivatives are known for their applications in the field of liquid crystals, semiconductor devices, electro-photography, molecular electronics, electro catalytic reagents, and also in the field of opto-electronics, electronics, and photonics.Solubility and variedphysico-chemical properties related with metal phthalocyanines were primarily depended upon the central metal atom, as well as the molecule’s peripheral position.
In this paper we have discusses the synthesis, spectral, magnetic susceptibility on symmetrically substituted transition metal (ΙΙ) tetra-(thiophene-2-yl) methaniminephthalocyanines.The available procedure is relatively modified and used for the synthetic route in the literature. The substituted transition metal (ΙΙ) tetra-1-(thiophene-2-yl) methaniminephthalocyanine complexes is given as per the scheme 1.
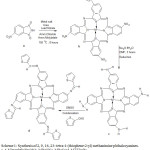 |
Scheme 1: Synthesis of 2, 9, 16, 23- tetra-1-(thiophene-2-yl) methanimine phthalocyanines. a. 4-Nitrophthalimide b. MPcONc. MPcOAd. MTTImPc Click here to View scheme |
Experimental
4-Nitrophthalimide were procuredfrom Sigma Aldrich. All the other chemicals were purchase from different chemical supplier. The substituted transition metal (II) tetraamino phthalocyanines complexes condensed into iminophthalocyanine complexes with 2-thiophenecarboxaldehyde compounds as shown in scheme-1.
Synthesis of Cobalt (II) tetra-1-(thiophene-2-yl) methaniminephthalocyaninecomplex.
4-nitro phthalamide (1 eq), cobaltsulphate (0.26 eq), and ammonium molybdate (0.5 eq) were taken with 12 mL of nitrobenzene, catalytic amount of ammonium molybdate and excess of urea in 3 necked round bottom flask. Reaction was kept for 120°C for first one hour and then gradually increased the temperature for every 30 minutes and finally, the mixture was heated to maintain180°C for5 hours to form tetranitro cobaltphthalocyanine.
In the next step thus formed Tetra nitro cobaltphthalocyanine (1 g eq.) and (8 g eq.) of sodium sulfide were taken with Dimethyl formamide in a 100 ml round bottomed flask and kept for stirring at 80oC for 24 hrs. Themixture was poured into ice cold water and theprecipitatewas formed, filtered off and washed with hot water several times to remove unreacted sodium sulfide. Then it was given a 0.1N HCl wash several times followed by washing repeatedly with 0.1N NaOH. Finally the precipitate is washed with hot H2O, filtered and dry in oven.
The finely powderedcobalt(II) tetraaminophthalocyanine (M-PcTA) (6.32 g 0.01 mole) was dissolved in dimethyl sulfoxidewith stoichiometric ratio of 2-thiphenecarboxaldehyde (12.6 mL,0.01 mole). In the catalytic quantity of conc.H2SO4 the mixture got refluxed for 5 hrs. Then mixture were poured in to an ice-cold water .The precipitate was washed with alcohol until several times to formed completely free from substituted aldehydes.The precipitate is washed with distilled hot water and dried in vacuum P2O5. Similar procedure is adopted to prepared the CuTTImPc, NiTTImPc, ZnTTImPc complexes.
Methods
Elemental analysis was carried out at STIC,Kochi, Kerala, India.The Gouy magnetic balance was used to measure the magnetic susceptibility of tetra substituted metal (II) iminomethane phthalocyanines at room temperature. Mercury cobalt(II) tetrathiocynaideas calibrant used reference complex to calculate diamagnetic behavior. SHIMADU UV- Visible spectrometer, UV-160A used for recording electronic absorption spectra. FT- IR spectral was used to identify the different functional groups in compounds.The diffraction patterns was recorded by using Phillip PW1710 XRD. Perkin Elmer thermal analyzer at a heating rate of 10°/ min both in the nitrogen atmosphere and air to calculate the TGA data.
Results and Discussion
Tetra-1-(thiophene-2-yl) methanimine phthalocyanines complexes are synthesis by adopting simple modified procedure. The colour of the complexes is bluish green in nature. It is soluble in DMF, DMSO, conc. H2SO4, pyridineand partially soluble in ethanol. The percentage of carbon, hydrogen, nitrogen, sulphur are agreed with theoretical value indicating the purity of the synthesized complexes. The proposed structure of the synthesized Tetra-1-(thiophene-2-yl) methanimine phthalocyanines complexes are shown in scheme-1and figure 1.
![Figure 1: Proposed structure of substituted metal (II) 2, 9 16, 23 -(thiophene-2-yl) methanimine phthalocyanine complexes. M=[ Cobalt, Copper, Nickel, Zinc]salt](http://www.orientjchem.org/wp-content/uploads/2018/06/Vol34No3_Syn_Fas_fig1-150x150.jpg) |
Figure 1: Proposed structure of substituted metal (II) 2, 9 16, 23 -(thiophene-2-yl) methanimine phthalocyanine complexes. M=[ Cobalt, Copper, Nickel, Zinc]salt |
Electronic [UV-Visible]
The concentration range at 1.0-1.5 X10-4 M in DMF, the electronic spectra of M-TTImPcs was recorded figure 2 and table 1.The bluish color of the phthalocyaninecompoundobserved due to a2u → eg & b2u → eg transitions state. The corresponding parent metal phthalocyanines are lower than the all complexes shows in the wavelength range721-734 nm. In all the complexes Q-band was doubled by ranging 572-588 nm. And the a1u → eg transition state of the phthalocyanine moiety was attributed with the originated Q-band both the sharp intense B-band ranged 325- 332 nm and weak L-band ranged 211- 219 nm was observed forall the complexes these B-band and L-band may be accounted for C-band in the phthalocyanines moiety.
 |
Figure 2: Electronic Spectra of a) Co-TTImPc,b)a) Cu-TTImPc,c) Ni-TTImPc,d) Zn-TTImPc |
Infra-red
Relevant Infra-red data was recorded by using KBr pellets, results are reported in table 1, figure 3. An intense (sharp) peak in the range 1619- 1630 cm-1 is due to C=N of imine group. C-N Ar.stretchingvibration peak in the range of 1365- 1388 cm-1. The C-S-C asy.stretching vibration is assigned in the range of 768 – 643 cm-1and C-S-C sym.stretching vibrations is observed in the range 1058- 1118cm-1. The peak is assigned in the range 3424- 3450cm-1 hydrogen bonding due to nitrogen atom in the phthalocyanines moiety and hydrogen atom in the KBr pellets. A weak signal C-H vibrational stretching at the periphery of the phthalocyanines moiety is assigned at the range 2325-2366cm-1. The range of 551-590 cm-1areattributedto various characteristic skeletal peak of the phthalocyanines molecule.
Table 1: Electronic and IR spectra data
| Complex Name | Molecular formula | UV-visible absorption | Elemental analysis (%) | IR-Spectral |
| Yield (%) (Colour) | (Molecular weight) | λ nm (log€) | found (calcd) | Data (cm-1) |
| Co-TTImPc | C52H28N12S4Co | 215 (4.90) | C, 61.90; (61.97) | 590, 768, 1058, 1124, 1378, 1619, 1709, 2366, 3450. |
| -85% | -1006.93 | 264 (4.79) | H, 2.68; (2.78) | |
| (Dark green) | 328 (5.39) | N, 16.54; (16.68) | ||
| 588 (5.07) | S, 12.67;(12.71) | |||
| 734 (5.11) | Co (5.85) | |||
| Cu-TTImPc | C52H28N12S4Cu | 211 (4.87) | C, 61.54; (61.68) | 582, 670, 1090, 1385, 1630, 1714, 2365, 3442. |
| -84% | -1011.55 | 259 (4.76) | H, 2.67; (2.76) | |
| (Dark green) | 332 (5.40) | N, 16.53; (16.60) | ||
| 577 (5.01) | S, 12.60;(12.65) | |||
| 732 (5.12) | Cu (6.28) | |||
| Ni-TTImPc | C52H28N12S4Ni | 214 (4.78) | C, 61.91; (61.98) | 573, 663, 1112, 1365, 1402, 1626, 2221, 2360, 3424. |
| -83% | -1006.69 | 262 (4.59) | H, 2.70; (2.78) | |
| (Dark green) | 325 (5.02) | N, 16.62; (16.68) | ||
| 580 (4.52) | S, 12.68;(12.71) | |||
| 724 (4.59) | Ni (5.82) | |||
| Zn-TTImPc | C52H28N12S4Zn | 219 (5.21) | C, 61.54; (61.57) | 551, 643, 1118, 1388, 1627, 2325, 2362, 3441. |
| -80% | -1013.39 | 251 (5.40) | H, 2.71; (2.76) | |
| (Dark green) | 330 (5.47) | N, 16.52; (16.57) | ||
| 572 (5.39) | S, 12.60;(12.63) | |||
| 721 (5.04) | Zn (6.45) |
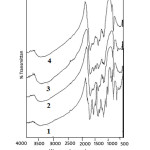 |
Figure 3: IR Spectra of 1) Co-TTImPc, 2) Cu-TTImPc, 3) Ni-TTImPc, 4) Zn-TTImPc |
Magnetic susceptibility
At an ambient temperature,magnetic measurement dataare presented in table 2. As per the magnetic measurement studiesCuTTImPc, CoTTImPc are behaves as paramagneticcomplexes and likewise NiTTImPc, ZnTTImPc are diamagnetic complexes in nature. The magnetic moment measured are higher the spin value in CuTTImPc and CoTTImPc compare to corresponding unpaired electron.By the crystallographic studies substituted phthalocyanines different metal are isomerphous and have sq. planar structure with D4h symmetry in fig-4.
Table 2: XRD and magnetic susceptibility measurements.
| Complex Name | 2θ angle (d Å) | (Relative Intensity) (%) | Field strength (KGauss) | Magnetic measurement χm x 10-6 cgs units | μeff(B.M) |
| 9.32 (10.32) | 100 | 2.2 | 3265.87 | 2.73 | |
| CoTTImPc | 28.81 (4.07) | 91.36 | 2.66 | 3035.41 | 2.55 |
| 24.43 (2.43) | 89.16 | 3.1 | 2838.16 | 2.41 | |
| 3.58 | 2713.64 | 2.34 | |||
| 4.01 | 2540.26 | 2.19 | |||
| 6.80 (12.79) | 100 | 2.2 | 3145.62 | 2.69 | |
| CuTTImPc | 8.58 (10.82) | 92.27 | 2.66 | 3003.16 | 2.57 |
| 29.92 (3.47) | 90.45 | 3.1 | 2851.79 | 2.43 | |
| 3.58 | 2702.61 | 2.31 | |||
| 4.01 | 2514.19 | 2.17 | |||
| 6.89 (14.72) | 100 | 2.66 | |||
| NiTTImPc | 8.82 (10.17) | 89.69 | –747.51 | — | |
| 27.26 (3.47) | 84.24 | ||||
| 6.88 (14.57) | 100 | 2.66 | |||
| ZnTTImPc | 8.79 (11.18) | 92.78 | -1041.02 | — | |
| 25.94 (2.83) | 94.15 |
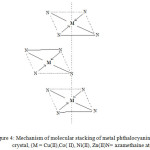 |
Figure 4: Mechanism of molecular stacking of metal phthalocyanine crystal, (M = Cu(II),Co( II), Ni(II), Zn(II)N= azamethaine atom |
Powder XRD
The range of 2θ angles were used take the powder XRD pattern of MTTImPcs. Angles6-70o showedthe relatively very poor crystalline peaks Table 2. The pattern is observed to same as the unsubstituted phthalocyanine complexes except the peak with diffuse intensity. Because of the molecular periphery there was a broadening in pattern. This broadening gives the hindrance due to effective stacking of the molecule. It is poor crystalline in nature phthalocyanine complexes.
Thermogravimetric and kinetic studies
In the Table 3, the thermo gravimetric analysis data are reported. In the two steps, an occurrence of the decomposition of above complexes was found. The first step indicated all the subsitutent groups in phthalocyanines are degradation in air at the temperature 250 to 350ºC. In the second step degradation steps all the complexes observed the major mass loss at 350- 600ºC. After thermal decomposition the residue remains as a metal oxide. In nitrogen atmosphere the substituted metal imino phthalocyanines complexes are very slow thermal decomposition.The 64 % that was decomposed at 700 ºC complex CoTTImPc, while for CuTTImPcmet with 58 % mass loss, NiTTImPc with 55 % and ZnTTImPc with 48 % mass loss. In air the complexes are shows the higher stability like CoTTImPc > CuTTImPc > NiTTImPc > ZnTTImPc. Boride’s method was used to evaluate the kinetic and thermodynamic parameters. And all the degradation steps are exothermic in nature as per DTA results.
Table 3: Thermodyamic degradation pattern.
| Complex Name | Weight Loss (%) | |||
| Decomposition [°C] | Found | Calcd | Fragmentation | |
| CoTTImPc | 250-350 | 28.84 | 30.04 | 4-Imino groups |
| 350-600 | 65.46 | 65.83 | Phthalocyanine moiety | |
| CuTTImPc | 250-350 | 28.82 | 30.15 | 4-Imino groups |
| 350-600 | 65.96 | 65.01 | Phthalocyanine moiety | |
| NiTTImPc | 250-350 | 28.79 | 30.19 | 4-Imino groups |
| 350-600 | 65.87 | 65.19 | Phthalocyanine moiety | |
| ZnTTImPc | 250-350 | 28.64 | 30.1 | 4-Imino groups |
| 350-600 | 65.52 | 65.11 | Phthalocyanine moiety |
The loss of periphery-tetra-1-(thiophene-2-yl) methanimine phthalocyanine complexes by the energy of activation (Ea) between the range of 0.87- 4.79 KJ/mole.The breaking of the main phthalocyanine complex ring takes place with the immediate removal of periphery substituents. By the negative entropies of the degradation, exothermic behavior of degradation was clearly indicated. Even there entropies are negative from -158.64 to -176.16 KJ.It was clearly indicated decomposition that the removal of functional groups. By using standard equations enthalpy, entropy and free energy was computed in table 4.
Table 4: Kinetic and thermodynamic data in inert atmosphere and air.
| Complex Name | Ea | Frequency factor. | Enthalpy | Entropy | Free energy |
| (kJ mole-1) | (lnA min-1) | (kJmole-1) | (kJ mole-1) | (kJ mole-1) | |
| CoTTImPc | 4.79 | 5.2 | -1.69 | -164.03 | 62.45 |
| I | -0.75 | -3.03 | (-1.27) | (-160.10) | -61.56 |
| II | 4.46 | 4.39 | 2.74 | -152.07 | 81.59 |
| -1.51 | -5.47 | (-1.41) | (-149.67) | -79.52 | |
| CuTTImPc | 0.87 | 6.34 | -1.37 | -176.16 | 74.86 |
| I | -0.68 | -3.35 | (-1.14) | (-174.11) | -74.69 |
| II | 4.64 | 7.39 | 2.79 | -143.82 | 79.42 |
| (1.25 | -4.65 | (-1.32) | (-141.89) | -77.24 | |
| NiTTImPc | 1.43 | 5.2 | -1.21 | -158.64 | 72.79 |
| I | (0.71) | -2.22 | (-0.74) | (-157.49) | -70.48 |
| II | 5.86 | 4.42 | -0.85 | -149.75 | 81.66 |
| (1.53) | -4.82 | (-0.83) | (-143.64) | -80.23 | |
| ZnTTImPc | 1.92 | 3.36 | -0.95 | -175.84 | 75.42 |
| I | (0.63) | -2.43 | (-1.50) | (-173.41) | -74.75 |
| II | 8.53 | 7.78 | 2.74 | -142.51 | 82.48 |
| (5.57) | -6.46 | (-1.53) | (-140.67) | -80.92 |
(I & II indicated as degradation in nitrogen atmosphere)
Conclusion
Synthesis of substutited metal phthalocyanines was adopted by simple modified method. The Cu and Cobalt phthalocyanine complexes are paramagnetic in behavior measured by magnetic susceptibility studies. The variation of magnetic moments with magnetic field [2.20 to 4.01 KG] clearly indicated the presence of intermolecular co-operative effect in the compound.These complexes are poor in crystanillity in nature.The stability of the complexes in gravimetric analysis in an inert atmosphere like CuTTImPc > CoTTImPc > NiTTImPc > ZnTTImP.
Acknowledgments
I sincerely thank to my guide KRV and HOD of chemistry, Manipal Institute of Technology, Manipal. Academy of Higher Education (MAHE), Manipal, Udupi for providing the laboratory facilities.
References
- Achar, B.N.; Fohlen.; Parker, G.M.; Keshavayya, J.; Polyhedron. 1989, 6(6), 1463.
CrossRef - Achar, George, M.; Fohlen; Parker, J.A. united state patent.1984, (4)45, 268.
- Arthur I. Vogel, Quantitative Iorganic Analysis, 3rd Ed., Longmans Publishers, London. 1964.
- Bartoli, J.F.; Brigoud, P.; Battioni,P.; Mansuy,D.; J. Chem. Soc, Chem.Commun.1994, 440.
- Broido, A. J. Polym. Sci. Part A-2.1969, 7 1761.
- Dandridge, A.E.; Drescher, H.A.; J. Thomas, J.(To Scottish Dyes Ltd) British patent. 1929, 822, 169.
- Dent, C.E.; Linstead, R. P.; Lowe, A. R. J. Chem. Soc. 1934,1033.
CrossRef - Eltis, R.B.; Lyons, J.E. Coord. Chem. Rev.1990, 105.
- Grinstaff, M.W.; Hill, M.G., Labinger, I.; Gray, H.B. Science.1994, 264, 311.
CrossRef - Harsans, M.C.; Guisinger, N.P.; Lyding, J. W. Nanotechnology.2000, 11, 70.
CrossRef - Jasinski,R. Nature.1964, 201, 1212.
CrossRef - Kobel,W.; Hanack, M. Inorg.chem. 1968, 25, 103.
CrossRef - Leznoff, C.C.; Lever, A.B.P. Phthalocyanines, properties and applications: VCH publications, Inc., New York, 1989, 1, 173.
- Manjunatha, N.; Imadadulla, M.; Lokesh,K.S.; Reddy.K. R.V. Dyes and Pigments.2018, 153, 213- 224.
CrossRef - Moinuddin Khan, M. H.; Fasiulla.;Venugopala Reddy, K.R.; Harish, M.N.K.; Keshavayya,J.J. Coord. Chem. 2007, 60(12), 1255-1267.
CrossRef - Paquette, B.; Ali, H.; Van Lier, E. J.J. Chim. Phys. Phys-Chim. Biol. 1991, 88, 1113.
CrossRef - Priola, S.A.; A. Raines,A.; Caughey, W.S. Science. 2000, 287, 1503.
CrossRef - Selwood, P. Magnetochemistry, New York, Interscience. 1956.
- Scblewein, D.; Karmann, E.; Ockermann, T.; Yanagi, H.; Electrochim. Acta.2000, 45, 4697.
CrossRef - Sheldon, R.A. Metalloporphyrin catalytic oxidation. Marcel Decker, New York, 1194.
- Somashakarappa, M.P.; Keshavayya. J. Spectrochemica Act. Part-A.2003, 59, 767.
- Somashekarappa, M P.; Venugopala Reddy, K R.; Harish, M.N.K.; Keshavayya, J.J. Molecular Structure.2005, 753, 190-194.
CrossRef - Somashekarappa, M. P.; Keshavayya, J. Synth. React. Inorg. Met-org. chem.1999, 29 (5), 767.
CrossRef - Somashekarappa, M.P.; Keshavayya,J. J. Soudi. Chem. Soc.1999, 3(2) 113.
- Somashekarappa, M.P.; Venugopala Reddy, K.R.; Harish, M.N.K.; Keshavayya, J. J. T. R. Chem.2004, 11(1), 1.
- Venugopala Reddy, K R.; Keshavayya, J. Synth React Inorg Met-Org Chem.2002, 32(7), 1235-1244.
CrossRef - Venugopala Reddy, K R.; Keshavayya, J. Turk J Chem. 2002, 26(4), 573.
- Whorle, D. Adv. polym. Sci. Review Article.1983, 50.

This work is licensed under a Creative Commons Attribution 4.0 International License.










