Studies on Phytoconstituents and Biological Potential of Stem of Moringa Oleifera
Department of Chemistry and Biochemistry, College of Basic Sciences and Humanities, Chaudhary Charan Singh Haryana Agricultural University, Hisar-125004, Haryana, India.
Corresponding Author E-mail: jyotipunia11@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340352
Article Received on : March 03, 2018
Article Accepted on : May 10, 2018
Article Published : 31 May 2018
Stem of Moringa oleifera were collected, shadow dried and chopped into small pieces. These were then extracted using methanol by refluxing method and the crude extract obtained was divided into two parts. One part was subjected to column chromatography which afforded a total of four compounds, namely, Cholest-5-en-3-ol(I), Stigmasterol(II), Gamma-sitosterol(III) and Tricosanoic acid(IV). All these compounds were first time reported from stem of Moringa. The other part of the crude extract was fractionated using different polarity solvents viz, hexane, benzene, chloroform, ethyl acetate, acetone and water. These fractions and methanol extract were then evaluated for antifungal activity by poisoned food technique against two phytopathogenic fungi. All the fractions were found to be more active against Rhizoctonia solani and Fusarium oxysporum.
KEYWORDS:Fusarium Oxysporum; Moringa Oleifera; Rhizoctonia Solani
Download this article as:| Copy the following to cite this article: Punia J, Singh R. Studies on Phytoconstituents and Biological Potential of Stem of Moringa Oleifera. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Punia J, Singh R. Studies on Phytoconstituents and Biological Potential of Stem of Moringa Oleifera. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=46352 |
Introduction
Moringa oleifera commonly known as ben oil tree or drumstick tree is a small sized tree native to India, Africa and Arabia.1 It is well known for its medicinal value as its all parts are used in the medicine. Roots and fruits are antiparalytic.2 The roots are also laxative, expectorant, diuretic and good for inflammations, throat, bronchitis, piles and cures stomatitis, urinary discharges and obstimate asthma.3 The root juice of Moringa when mixed with milk helps to cure lower back pain as it contains analgesics called Moringine and Moringinine.4 Its roots are also prescribed for the treatment of snakebites and scorpion stings. Its leaves are antihelmentic, aphrodisiac and cure hallucinations, dry tumors, hiccough and asthma.5 Moringa flowers are a rich source of Potassium and Calcium. They are also used in the folk remedies for tumors. They also act as hypocholesterolemic and antiarthritic agents and helps to cure urinary problems and cold.6 Immature pods are good source of palmitic acid, linoleic acid, linolenic acid and oleic acids. So they can be used in the diet of an obese person.7 The medicinal value of this plant was long been used in folk remedies and was attributed to the presence of various bioactive compounds in its different parts. But the stem of Moringa oleifera remained unexecuted by the scientific community for its bioactive components as well as for its bioevaluation. So, the relevance and innovation of this study lies in the isolation, characterization and biological potential of Moringa oleifera stem.
Experimental
Sodium hydroxide, sodium silicate, Neseller’s reagent, 2,4-dinitrophenol(DNP), ammonium molybdate and ammonium vandate were purchased from CDH, Daryaganj, New Delhi. All the solvents used in this study were of analytical grade and purchased from CDH or SD Fine Chem Limited, Mumbai, India. The adsorbents used for chromatography were silica gel (60-120 mesh) and silica gel G and were obtained from Himedia Laboratories Pvt. Ltd., Nashik, Mumbai. 1H NMR spectra of the isolated compounds were recorded on Sophisticated multinuclear FT NMR Spectrophotometer model Avance-II (Bruker 400MHz). CDCl3 and DMSO were used as solvents. Chemical shifts were recorded in δ (ppm) using Tetramethyl silane (TMS) as an internal standard. LC-MS were recorded with a Waters Micromass Q-ToF Micro Mass spectrometer. It is equipped with electronspray ionization (ESI) and atmospheric pressure chemical ionization (APcI) source having mass range of 4000 amu in quadruple and 20000 amu in ToF. Melting points were determined with a Ganson Electrical Melting Point apparatus. IR spectra were recorded with a Perkin Elmer Spectrum RX-I FTIR. It has a resolution of 1cm-1 and scan range of 4000 to 250 cm-1.
Material and methods
Plant material
Stem of Moringa oleifera were collected from the campus of Chaudhary Charan Singh Haryana Agricultural University, Hisar, Haryana, India. These were washed thoroughly with water to remove dust, shadow dried and chopped into small pieces. These were then kept in air tight containers for further use.
Extraction and fractionation
The shadow dried, chopped pieces of stem of drumstick tree (2kg) were extracted with hot methanol by refluxing method for eight hours. The process was repeated thrice and the respective extractives were pooled together. The obtained extractives were evaporated on a rotary evaporator to give a crude extract (176g). This extract was further divided into two parts. One major part (100g) was mixed with silica gel (60-120 mesh size) and used to fill the column. The remaining part (76g) was further fractionated with different polarity solvents viz hexane, benzene, chloroform, ethyl acetate, acetone and water. Each obtained fraction was evaporated to give a crude mass and stored in a refrigerator till use. These fractions and methanolic extract were used for determination of antifungal activity (Fig. 1).
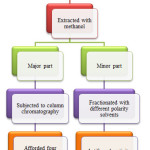 |
Figure 1: Extraction, fractionation and isolation scheme of stem of Moringa oleifera Click here to View figure |
Preliminary phytochemical screening
The freshly prepared methanolic extract of stem of Moringa oleifera was subjected to qualitative chemical tests using standard methods. This helps in the identification of various classes of bioactive chemical constituents.
Column chromatography
The methanolic extract of stem of Moringa oleifera which was mixed with silica gel 60-120 mesh size subjected to column chromatography. A glass column of 1000×40 mm size was packed with slurry of silica gel (60-120 mesh size) in hexane. A portion of the methanolic extract of stem was introduced onto the column and eluted with solvents of increasing polarity The elutropic series of solvents used was hexane, benzene, ethyl acetate, methanol and their mixtures. Each eluate obtained was monitored by using Thin Layer Chromatography plates. The column chromatography afforded four compounds labeled as I to IV.
Compound I Fractions (1-15) were repeatedly separated by silica gel column chromatography on elution with benzene: hexane (1:9). It was crystallized by using acetone and obtained as white needles (30mg) having melting point 144-146ºC. Its Rf value was found to be 0.42 in benzene. The molecular formula C28H48O was deduced from its GCMS having molecular mass 400.
1H NMR (δ, CDCl3) 0.88 (s, 3H, C18-CH3), 0.90 (s, 3H, C26-CH3), 0.97 (s, 3H, C27-CH3), 1.11 (s, 3H, C19-CH3), 1.15 (s, 3H, C21-CH3), 1.18 (s, 3H, C28-CH3), 1.24-2.88 (m, 26H, 10×-CH2, 6×-CH3), 3.60 (m, J= 4.0Hz, 1H, CH-OH), 5.34 (m, J= 8.0Hz, 1H, =CH). 13CNMR (δ, CDCl3) 141.80, 120.15, 71.56, 55.10, 50.15, 40.28, 38.20, 33.40, 32.75, 28.20, 28.10, 22.10, 14.10, 13.70.
IR
![]()
3419, 3322, 2955, 2927, 2852, 1462, 1378, 1259, 1055, 801, 759.
LC-MS (m/z, % intensity) 400 (78), 315.3 (80), 255.2 (52), 213.2 (94), 145.1 (100), 107.1 (82).
Compound II was obtained on elution with benzene: hexane (1:9). Fractions 16-26 were separated by silica gel column chromatography and were pooled together after observing their TLC. The compound was crystallized from benzene (25mg). The purity of the compound was confirmed by Thin Layer Chromatography. The melting point of this compound was found to be 167-168ºC. Rf value was found 0.48 in ethyl acetate: benzene (1:19). The molecular formula C29H48O deduced from its GC-MS having mass 412.4.
1H NMR (δ, CDCl3) 0.74 (s, 6H, 1×C29-CH3 and C26-CH3), 0.97(s, 6H, 1×C24-CH3 and 1×C27-CH3), 1.17 (s, 3H, 1×C28-CH3), 1.21 (s, 3H, 1×C19-CH3), 3.78 (d, J=4.0Hz, 1H, 1×C3-CH), 4.38 (s, 1H, 1×C23-CH), 4.86 (m, J=16.0Hz, 1H, 1×C22-CH), 5.03 (m, J=4.0Hz, 1H, 1×C6-CH), 7.28 (s, 1H, 1× -OH).
IR
![]()
3419, 3322, 2955, 1462, 1368, 1055, 801, 759,667.
GC-MS (m/z, relative abundance) 412.4(60), 351.3(25), 300.2(38), 255.2(77), 213.2(57), 159.2(100),83.1(95).
Compound III was obtained as colorless crystals (45mg) on elution with benzene: hexane (1:9). It was recyrstallised from ethanol. Its melting point was found to be 145-147ºC. The molecular formula C29H50O was deduced from its GC-MS analysis.
1H NMR (δ, CDCl3) 0.68 (s, J=8.0Hz, 3H, 1×C18-CH3), 0.79(d, J=4.0Hz, 6H, 1×C26-CH3 and 1×C27-CH3), 0.83 (t, J=8.0Hz, 3H, 1×C21-CH3), 0.90(d, J=4.0Hz, 3H, 1×C29-CH3), 1.24 (s, 3H, 1×C19-CH3), 5.12(m, 1H, -OH), 5.32 (br, J=4.0Hz, 1H, C6-CH).
IR
![]()
3419, 2955, 2852, 1659, 1462, 1378, 1055, 801, 759.
GC-MS (m/z, relative intensity) 414.4(80), 329.3(68), 303(41), 329.3(67), 255.2(65), 213.2(98), 145.1(100), 107.1(85).
Compound IV was isolated on elution with benzene: hexane (1:1) and crystallized out from benzene to get creamish salt like granules, 30mg, melting point 70-74ºC. The compound IV responded to brown color in Libermann-Burchard’s reaction. Its Rf value was found to be 0.11 in benzene. The molecular formula C23H46O2 was deduced from m/z 355 by it GC-MS.
1H NMR (δ, CDCl3) 0.88 (t, J=8.0Hz, 3H, 1×-CH3), 1.26 (br, J=4.0 Hz, 38H, 19×-CH2), 1.63 (m, J=8.0Hz, 2H, 1×-CH2-CH2COOH), 2.34 (t, J=8.0 Hz, 2H, 1×-CH2COOH).
IR
![]()
2917, 2849, 1706, 1471, 1463, 1432, 1297, 1116, 939, 719.
GC-MS (m/z, relative intensity) 355.2 (5), 267.1 (10), 149.1 (6), 84(100).
Biovaluation
Test organism The antifungal activity of different extracts/ fractions of stem of Moringa oleifera was investigated using two phytopathogenic fungi i.e. Rhizoctonia solani and Fusarium oxysporum which were obtained from the Department of Plant Pathology, College of Agriculture, Chaudhary Charan Singh Haryana Agricultural University, Hisar, Haryana, India. The fungal isolates were allowed to grow on Potato Dextrose Agar8 (PDA) at 25±2ºC until they sporulated. A 4-6 day old culture of each fungus was used for testing antifungal activity.
Bioassay
Poisoned Food Technique9 was used for determination of antifungal activity of different solvent fractions and extract of Moringa oleifera stem. Two sets were maintained- one for the treatment and another for control. The treatment set at different concentrations viz 250, 500, 1000 and 2000 µg/ml was prepared by mixing the required quantity (25, 50, 100 and 200 mg respectively) in 1ml of DMSO and then added pre-sterilized PDA. In control set, 1ml DMSO was mixed with PDA. These treatments and control were then poured in pre-sterilized Petri plates and allowed to solidify at room temperature. After solidification, mycelia disc of 5mm diameter cut out from 4-6 day old culture of test fungi were aseptically placed in Petri plates of different treatment and control sets. The Petri plates were then wrapped with para film along the rim to prevent contamination. The inoculated plates were then inverted and incubated at 25±2ºC and the observations were recorded when the control plate got completely filled with test fungus. Colony diameter was determined by measuring the average radial growth of each plate. The data recorded in each case was mean of three replicates. The fungal growth inhibition (%) was calculated by using the following formula:
![]()
Where C = mycelia growth in control plate, T = mycelia growth in treated plate
The concentration of plant extract/ fractions producing 50% growth inhibition (EC50) was calculated using SPSS Statistics 19 software.
Data Analysis
All the experimental measurements were carried out in triplicate and results were presented as mean± standard deviation. One way and two way analysis of variance (ANOVA) was carried out to assess any significant differences between the means (p<0.05) in Online Statistical Analysis (OPSTAT), CCS HAU, Hisar. EC50 values of antifungal activity were calculated using SPSS Statistics 19 software. All other measurements and calculations were carried out in Microsoft Excel 2007.
Results and Discussion
Preliminary Phytochemical Screening
Methanolic extract of stem of M. oleifera was screened for various phytochemicals like saponins, tannins, phytosterols, terpenoids, flavonoids, etc. The major phytochemicals found present were saponins, carbohydrates, anthraquinone glycosides, alkaloids, flavonoids, terpenoids, phytosterols, proteins and amino acids which are shown by positive (+ive) sign in table 1 while tannins and cardiac glycosides were found to be absent and shown by negative sign (-ive).
Table 1: Preliminary phytochemical screening of methanolic extract of stem of Moringa oleifera
|
Sr. no. |
Phytochemicals tested | Name of the test |
Result |
|
1. |
Saponins | Frothing test |
+ive |
|
2. |
Tannins | Ferric chloride test |
-ive |
|
3. |
Carbohydrates | Fehling’s test, Tollen’s reagent test |
+ive |
|
4. |
Cardiac glycosides | Keller-Killiani test |
-ive |
|
5. |
Anthraquinone glycosides | Hydroxyanthraquinine test |
+ive |
|
6. |
Alkaloids | Hager’s test |
+ive |
|
7. |
Flavonoids | Alkaline reagent test |
+ive |
|
8. |
Terpenoids | Salkowski test |
+ive |
|
9. |
Phytosterols | Liebermann- Burchard’s test |
+ive |
|
10. |
Protein | Biuret test |
+ive |
|
11. |
Amino acids | Millon’s test |
+ive |
+ive shows the presence while –ive shows the absence.
Isolation and Characterization of Isolated Compounds
Compound I, (Cholest-5-en-3-ol)
Compound I was obtained on elution with benzene: hexane (1:9). Its Rf value was found to be 0.42 in benzene. Its melting point was found to be 144-146ºC which is in agreement with the literature data 148-150ºC.10 GC-MS analysis of compound I showed that its molecular mass is 400 with molecular formula C28H48O. The IR spectra of the compound showed that it does not possess a carbonyl group which is confirmed by absence of absorption bands ranging from 1725-1810 cm-1. The absorptions ranging from 660-1100 cm-1 are characteristic absorptions of steroids. The absorption bands at 3419 and 3322 cm-1 indicated the presence of hydroxyl group.
The 1H NMR of compound I in CDCl3 exhibited a multiplet at 5.34δ attributed to coupling of the H-atom which is bonded to sp2 carbon atom (H-6) with hydrogen atoms on adjacent CH2 group. A multiplet at 3.60δ was assignable to a proton (H-3) bonded to –OH group. Six singlets of methyl functionality integrating for three protons each appeared at 0.88, 0.90, 0.97, 1.11, 1.15 and 1.18δ. Another multiplet observed ranging from 1.24-2.88δ corresponds to the remaining ten methylene and six methine protons in cholest-5-en-3-ol. All the above spectral data is in agreement for the following structure which corresponds to cholest-5-en-3-ol. This is the first report of isolation and characterization of cholest-5-en-3-ol from stem of Moringa oleifera.
Table 2: Antifungal activity (%) and EC50 values (µg/ml) of various extract/ fractions of stem of Moringa oleifera against Rhizoctonia solani
|
Sr. No. |
Extract/ Fractions |
Percentage Growth Inhibition |
EC50 (µg/ml) |
|||
|
250µg/ml |
500 µg/ml |
1000 µg/ml |
2000 µg/ml |
|||
|
1. |
Hexane |
30.98±1.80 |
37.06±0.59 |
41.37±0.34 |
49.41±1.18 |
2295.65 |
|
2. |
Benzene |
32.94±0.00 |
38.24±0.59 |
48.43±0.34 |
60.00±1.02 |
1044.47 |
|
3. |
Chloroform |
41.37±0.34 |
43.33±0.68 |
47.45±2.07 |
52.35±2.12 |
1500.32 |
|
4. |
Ethyl acetate |
36.67±1.48 |
41.76±0.59 |
48.24±0.59 |
66.27±0.34 |
804.14 |
|
5. |
Acetone |
21.57±1.48 |
30.98±0.90 |
36.86±0.34 |
61.76±0.59 |
1378.48 |
|
6. |
Water |
5.29±0.59 |
12.94±0.59 |
16.47±0.59 |
45.29±1.18 |
1378.48 |
|
7. |
Methanol |
16.08±2.38 |
23.33±1.89 |
41.57±0.34 |
68.43±1.48 |
1178.82 |
|
Factors |
SE(d) |
CD at 5% |
||||
|
Concentration |
0.352 |
0.706 |
||||
|
Compound |
0.465 |
0.934 |
||||
|
Conc. × Compound |
0.930 |
1.868 |
||||
All the values are mean±S.D.
Mean of three replicates was taken (n=3).
µg/ml means microgram per millilitre.
EC50 means inhibition concentration at which 50% of the growth is inhibited.
Compound II, (Stigmasterol)
Compound II was obtained on elution with benzene: hexane (1:9). Its Rf value was found to be 0.48 in ethyl acetate: benzene (1:19). Its melting point was found to be 167-168ºC (literature m.p. 169.5ºC).11 The absorptions at 3419 and 3322 cm-1 in its IR spectra confirmed the presence of –OH functionality in its structure. The molecular formula C29H48O was deduced from its GC-MS with molecular mass 412.4.
In 1H NMR spectra of the compound in CDCl3, a singlet at 0.74δ indicated the presence of six protons of two methyl groups. Six protons of another two methyl groups could be picked up at 0.97δ as a singlet. Two singlets appeared at 1.17δ and 1.21δ for two methyl groups positioned at C19 and C28. A doublet at 3.78δ with coupling constant (J=4.0Hz) integrating for one proton assignable to –CH moiety attached to –OH group at C3 position. A singlet centred at 4.38δ for one proton was assignable to hydrogen at C23. 1HNMR spectra displayed a multiplet at 4.86δ integrating for one proton at C22. A singlet at 7.28δ integrating for one proton confirmed the presence of hydroxyl group in it. The spectroscopic data was in agreement with the literature data12 of stigmasterol. This is the first report of isolation and characterization of this compound from Moringa oleifera stem.
Table 3: Antifungal activity (%) and EC50 values (µg/ml) of various extract/ fractions of stem of Moringa oleifera against Fusarium oxysporum
|
Sr. No. |
Extract/ Fractions |
Percentage Growth Inhibition |
EC50 (µg/ml) |
|||
|
250µg/ml |
500 µg/ml |
1000 µg/ml |
2000 µg/ml |
|||
|
1. |
Hexane |
5.29±0.59 |
16.27±0.34 |
20.98±0.34 |
22.94±1.02 |
4129.82 |
|
2. |
Benzene |
15.69±1.36 |
21.96±0.34 |
24.12±0.59 |
28.63±0.34 |
4664.56 |
|
3. |
Chloroform |
11.37±0.34 |
24.12±0.59 |
38.43±0.34 |
59.22±0.34 |
1457.17 |
|
4. |
Ethyl acetate |
11.57±0.34 |
20.20±0.90 |
27.45±0.68 |
30.20±0.68 |
3790.44 |
|
5. |
Acetone |
15.49±0.34 |
27.45±0.68 |
47.45±0.34 |
51.37±0.68 |
1537.03 |
|
6. |
Water |
4.12±0.59 |
11.57±0.34 |
17.25±0.68 |
21.18±1.18 |
4740.79 |
|
7. |
Methanol |
13.73±0.68 |
27.84±0.34 |
52.41±0.50 |
58.43±0.34 |
1196.90 |
|
Factors |
SE(d) |
CD at 5% |
||||
|
Concentration |
0.190 |
0.382 |
||||
|
Compound |
0.252 |
0.505 |
||||
|
Conc. × Compound |
0.503 |
1.011 |
||||
All the values are mean±S.D.
Mean of three replicates was taken (n=3).
µg/ml means microgram per millilitre.
EC50 means inhibition concentration at which 50% of the growth is inhibited.
Compound III, (Gamma-Sitosterol)
Compound III was obtained on elution with benzene: hexane (1:9) and crystallised out from ethanol, 45mg, melting point 145-147ºC (literature m.p. 147-148ºC13). It gave green color in Liebermann-Burchard reaction indicating the presence of steroids. GC-MS analysis of this compound suggested the molecular formula to be C29H50O and molecular mass 414. Its IR spectra gave absorption peak at 3419cm-1 indicating the presence of hydroxyl group in the compound. Other absorption peaks at 2955, 2852, 1659, 1462, 1378, 1055, 801 and 759 cm-1.
The 1H NMR spectra of the compound III in CDCl3 exhibited a singlet at 0.68δ for three protons which was assignable to methyl group present at C18 position. A doublet centered at 0.79δ with J=4.0 Hz integrating for six protons indicated two methyl groups positioned at C26 and C27. A doublet centered at 0.90δ with J=4.0 Hz integrating three protons suggested the presence of a methyl group at C21 and a triplet at 0.83δ (J=8.0 Hz) representing three protons was assignable to methyl group at C29 position. A singlet at 1.24δ representing three protons could be due to methyl group at C19 position. A multiplet centered at 5.12δ integrating for one proton could be of hydroxyl group. A broad signal at 5.32δ for one proton was assigned to an olefinic proton. The spectral analysis is in perfect agreement with the literature data of γ-sitosterol. From the literature survey, it seems that this is the first time report of gamma-sitosterol from stem of Moringa oleifera.
Compound IV, (Tricosanoic Acid)
Methanolic extract of stem of Moringa oleifera when subjected to column chromatography gave compound IV with benzene: hexane (1:1) solvent system. Total twenty nine fractions were collected and monitored by TLC. It was crystallised in benzene (30mg) and having melting point 70-74ºC. Its molecular formula, C23H46O2 was deduced from GC-MS with molecular mass 355. Absorptions at 1706cm-1 in IR spectra confirmed the presence of >C=O group in this compound. Other absorptions appeared at 2917, 2849, 1463, 1116 and 719cm-1.
The 1HNMR spectra of this compound showed a single peak at 7.26δ which represents the residual peak of CDCl3. The spectrum exhibited a triplet at 2.34δ for two hydrogens with J value 8.0 Hz adjacent to carboxylic acid group. A multiplet was observed at 1.63δ for methylene protons at C-3 position. A broad singlet appeared at 1.26δ for another methylene integrating for thirty eight protons. A sharp triplet at 0.88δ appeared for terminal methyl protons. The spectral data helped us to identify the compound IV to be tricosanoic acid. This is the first report of isolation and characterization of tricosanoic acid from stem of Moringa oleifera.
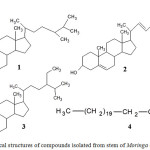 |
Figure 2: Chemical structures of compounds isolated from stem of Moringa oleifera
|
Bioevaluation
Antifungal Activity of Stem of Moringa Oleifera
Antifungal activity of different extract/ fractions of stem of M. oleifera against Rhizoctonia solani and Fusarium oxysporum are presented in figure 3 and 4. A perusal of the data showed that irrespective of the concentration, 2000 µg/ml concentration was found to be the most toxic and 250 µg/ml concentration was the least toxic. All the extract/ fractions of stem of drumstick tree were found to be more active against R. solani than F. oxysporum. Methanol extract was found to be the most active at 2000 µg/ml concentration with 68.43±1.48% growth inhibition against R. solani while in terms of EC50 value ethyl acetate fraction was found to be the most active with 804.14 µg/ml EC50 value. Against F. oxysporum, the maximum activity was shown by chloroform fraction at 2000 µg/ml concentration with 59.22±0.34% growth inhibition and 1457.17 µg/ml EC50 value. Minimum activity was shown by water fraction of stem of M. oleifera at 250 µg/ml concentration against R. solani and F. oxysporum. Moderate activity was shown by hexane, benzene, chloroform and ethyl acetate fractions against R. solani. While against F. oxysporum, moderate activity was shown by benzene, ethyl acetate and water fractions. A significant difference was found among all the fractions at different test concentrations. Critical difference values for antifungal activity of various extract/ fractions of stem of Moringa oleifera were calculated. Interaction of compounds and concentrations was statistically significant with a value 1.868 against R. solani and 1.011 against F. oxysporum for concentration × compound.
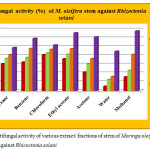 |
Figure 3: Antifungal activity of various extract/ fractions of stem of Moringa oleifera against Rhizoctonia solani |
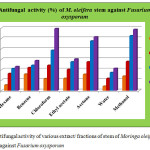 |
Figure 4: Antifungal activity of various extract/ fractions of stem of Moringa oleifera against Fusarium oxysporum |
Acknowledgements
Authors are thankful to SAIF, CIL and UCIL, Panjab University, Chandigarh for providing facilities for spectral analysis. We are also grateful to Department of Chemistry and Biochemistry and Department of Plant Pathology, CCS HAU, Hisar.
References
- Mughal, M. H.; Ali, G.; Srivasta, P. S.; Iqbal, M. Harmdad Med. 1999, 42, 37-42.
- Khare, C. P. Encyclopedia of Indian Medicinal Plants, Springer, Berlin, 2004.Kirtikar, K. R.; Basu, B. D. Indian Medicinal Plants. 1975, 1(2), 676–683.
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A. H. Phytother. Res. 2007, 21(1), 17-25.
CrossRef - Fuglie, L. J. The Moringa Tree: A Local Solution to Malnutrition. Published in Dakar, Senegal, 2005.
- Dhakar, R. C.; Maurya, S. D.; Pooniya, B. K.; Bairwa, N.; Gupta, M. Chron. Young Sci. 2011, 2(3), 119.
CrossRef - Sánchez-Machado, D. I.; Núñez-Gastélum, J. A.; Reyes-Moreno, C.; Ramírez-Wong, B.; López-Cervantes, J. Food Anal. Methods. 2010, 3(3), 175-180.
CrossRef - Tuite, J. Plant pathological methods, fungi and bacteria. Burgess Publishing Co., Minneapolis, Minn, 1969.
- Grover, R. K.; Moore, J. D. Phytopathology, 1962, 52, 876–880.
- Williams, J. H.; Kuchmak, M.; Witter, R. F. J.Lipid Res. 1965, 6(4), 461-465.Buckingham, J. Dictionary of Natural Products Supplement, CRC press, 1997, 4(11).
- Rajput, A. P.; Rajput, T. Int. J. Biochem. Chem. 2012, 6, 130-135.
CrossRef - Balamurugan, R.; Stalin, A.; Ignacimuthu, S. Eur. J. Med. Chem. 2012, 47, 38-43.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










