Vibrational Spectra of Copper Tetramesityl Porphyrin using Vibron Model
J. Vijayasekhar1 , M. V. Subba Rao2 and V. Sreeram3
, M. V. Subba Rao2 and V. Sreeram3
1Department of Mathematics, GITAM University, Hyderabad, India.
2Department of Mathematics, ANITS, Visakhapatnam, Andhra Pradesh, India.
3Department of Chemistry, A.G and S.G. Siddhartha Degree College, Vuyyuru, A.P, India.
Corresponding Author E-mail: vijayjaliparthi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340165
In this paper we calculated the fundamental level vibrational spectra of Metalloporphyrin (bio molecule) copper tetramesityl porphyrin (Cu(TMP)) using Vibron model.
KEYWORDS:Vibrational Spectra; Vibron Model; Metalloporphyrins; Cu(TMP)
Download this article as:| Copy the following to cite this article: Vijayasekhar J, Rao M. V. S, Sreeram V. Vibrational Spectra of Copper Tetramesityl Porphyrin using Vibron Model. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Vijayasekhar J, Rao M. V. S, Sreeram V. Vibrational Spectra of Copper Tetramesityl Porphyrin using Vibron Model. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42296 |
Introduction
Group theory is a well known tool that simplifies the process of obtaining a variety of information about molecules and their symmetries. Molecules are classified according to their symmetry properties and from that one can identify, the molecular symmetry point group. The molecular symmetry point group of Metalloporphyrins is D4h, which contains the principal Cn axis, n perpendicular C2 axis, and the horizontal plane of symmetry.
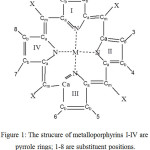 |
Figure 1: The strucure of metalloporphyrins I-IV are pyrrole rings; 1-8 are substituent positions. X positions are (=CH-) bridges Click here to View figure |
In 2008, Karumuri et al, applied Vibron model for coupled anharmonic oscillators to describe the stretching vibrations of medium size molecules and calculated vibrational spectra of nickel octaethyl Porphyrins for the stretching mode Cm – H. In the intervening years extended this model to calculate stretching vibrational frequencies of nickel tetraphenyl porphyrin and copper octaethyl porphyrin for different vibrational bands [1-9].
Vibron model for Metalloporphyrins
The general calculation procedure of vibrational spectra of Metalloporphyrin by Vibron model discussed here [10, 11]. The Hamiltonian for the polyatomic molecules is of the form
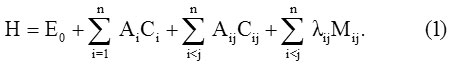
Here i vary from 1 to n for n stretching bonds and (Ai, Aij, λij)are algebraic parameters, which are determined by spectroscopic data. Where Ci is an invariant operator (uncoupled bonds) with eigenvalues
![]()
Diagonal matrix elements of the invariant operator Cij (coupled bonds) and diagonal and non-diagonal matrix elements of Majorana operator Mij obtained from the following relations,
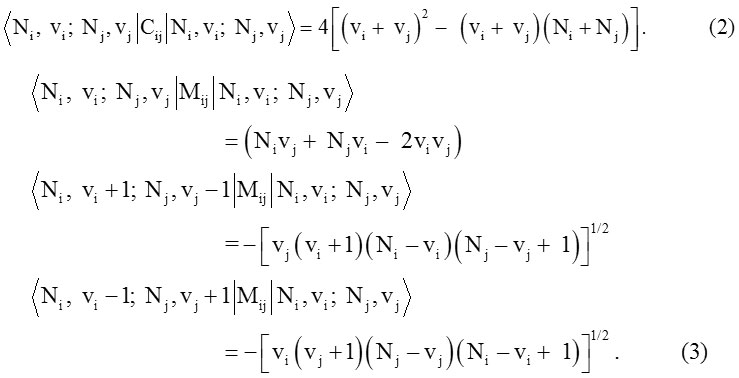
Where Vi (i = 1,2,3,…) are vibrational quantum numbers.
The vibron number Ni (i = 1,2,3,…) for stretching bonds of molecule will be calculated by the following relation
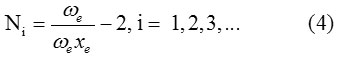
Where ωe and ωe xe are spectroscopic constants. The initial guess value for the parameter Ai is obtained by using the energy equation for the single-oscillator fundamental mode, which is given as,
![]()
Initial guess for Aij may be taken as zero. The parameter λij obtained from the relation
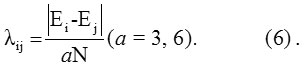
Results
Table 1: Vibrational spectra of Cu(TMP)
|
Symmetry Species |
Vibrational mode |
Vibrational frequencies (cm-1) |
|
A1g |
(Cm-C) |
1235.04348 |
|
B2g |
(Cm-C) |
1247.03267 |
|
Eu |
(Cm-C) |
1256.90364 |
|
A1g |
(Cb-H) |
1470.00431 |
|
B2g |
(Cb-H) |
1476.05321 |
|
Eu |
(Cb-H) |
1470.87451 |
Table 2: Algebraic parameters
|
Algebraic parameters |
Cm – C |
Cb – H |
|
A |
-2.19234 cm-1 |
-5.90923 cm-1 |
|
Aij |
-0.98232 cm-1 |
-2.92012 cm-1 |
| λij (a = 3) |
0.02421 cm-1 |
0.80834 cm-1 |
| λij (a = 6) |
0.20091 cm-1 |
0.03421 cm-1 |
|
N (Dimensionless) |
140 |
44 |
Conclusion
In this paper we have calculated the vibrational frequencies of copper tetramesityl porphyrin (Cu(TMP)) for the stretching modes (Cm-C) and (Cb-H).
References
- Karumuri, S. R. Indian J. Phys. 2012, 86(12), 1147-1153.
CrossRef - Karumuri, S. R.; Sekhar, J.V.; Sreeram, V.; Rao, V. U.M.; Rao, M.V.B.; J Mol Spectrosc. 2011, 269, 119-123.
CrossRef - Karumuri, S. R.; Srinivas, G.; Sekhar, J.V.; Sreeram, V.; Rao, V. U.M.; Srinivas, Y.; Babu, K.S.; Kumar, V.S.S.; Hanumaiah, A.; Chin Phys B. 2013, 22(9), 090304 (1-8).
- Karumuri, S. R.; Sarkar, N.K.; Choudhury, J.; Bhattacharjee, R.; Mol. Phys. 2008, 106(14), 1733-1737.
CrossRef - Karumuri, S. R.; Sarkar, N.K.; Choudhury, J.; Bhattacharjee, R.; Chin Phys Lett. 2009, 26(9), 093301 (1-4).
- Karumuri, S. R. Chin Phys Lett. 2010, 27(10), 103301 (1-4).
- Karumuri, S. R. J Mol Spectrosc. 2010, 259, 86-92.
CrossRef - Karumuri, S R. Eur. Phys. J. 2015, 69, 281.
- Karumuri, S. R.; Sravani, K.G.; Mol. Phys. 2016, 114(5), 643-649.
CrossRef - Iachello, F.; Levine, R. D. Oxford University Press, Oxford. 1995.
- Oss, S. Adv. Chem. Phys. 1996, 93, 455- 649.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









