Synthesis, Characterization and Cytotoxic Activity of new Indole Schiff Bases Derived from 2-(5-Chloro-3,3-Dimethyl-1,3-Dihydro-Indol-2-Ylidene)-Malonaldehyde with Aniline Substituted
Aseel Faeq Ghaidan1 , Fadhil Lafta Faraj2 and Zaynab Saad Abdulghany2
, Fadhil Lafta Faraj2 and Zaynab Saad Abdulghany2
1Department of Molecular biology, Iraqi center for cancer and medical genetic research, University of AL-Mustansiriyah, Iraq.
2Department of Chemistry, Faculty of Science, University of Diyala, Diyala Governorate, Iraq.
Corresponding Author E-mail: fadhillaftafaraj@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340119
Series of new compounds of indole Schiff base derivatives have been synthesized by reaction of 2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-malonaldehyde with aniline substituted. The chemical structures of the synthesized compounds were characterized by TLC, FT-IR, 1H, 13C NMR and APT 13C NMR. The in vitro anticancer activity of the new synthesized compounds tested against – AMJ breast cancer cell line. The revealed data showed that compounds have promising anticancer activity against AMJ13 cell line at low concentrations.
KEYWORDS:Schiff Bases; Aniline Substituted; Aldehyde; Cytotoxic Activity
Download this article as:| Copy the following to cite this article: Ghaidan A. F, Faraj F. L, Abdulghany Z. S. Synthesis, Characterization and Cytotoxic Activity of new Indole Schiff Bases Derived from 2-(5-Chloro-3,3-Dimethyl-1,3-Dihydro-Indol-2-Ylidene)-Malonaldehyde with Aniline Substituted. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Ghaidan A. F, Faraj F. L, Abdulghany Z. S. Synthesis, Characterization and Cytotoxic Activity of new Indole Schiff Bases Derived from 2-(5-Chloro-3,3-Dimethyl-1,3-Dihydro-Indol-2-Ylidene)-Malonaldehyde with Aniline Substituted. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=43270 |
Introduction
Schiff bases are important compounds in the chemistry and biochemistry fields due to their biological activities [1]. Schiff bases which are a class of compounds containing an azomethine group (-C=N-) as functional group have focus attention for a long time due to their medicinal and pharmaceutical activities [2].Schiff bases were first reported by Hugo Schiff in 1864, they are formed by the condensation reaction of aldehydes or ketones with primary amines in the presence of acid as a catalyst [3].They have many applications in different fields for example: antibacterial, antifungal, and antitumor activity [4].
Schiff bases obtain from different organic heterocyclic compounds chiefly those consisting of indole molecule, There is an important class of organic heterocyclic compounds because they have abroad applications for example, anti-oxidant, anticancer[5], Antibacterial, antifungal, anti-inflammatory and antiviral properties [6].
Cancer is a disease that make cells growth in the body out of control; the most common types of cancer are breast, lung, colon and prostate cancer [7].When it is start in the breast then it is called breast cancer (BC) [8].
BC is one of the most common diagnosed types of cancer in women around the world an inducing to cancer death with almost 1.67 million new cases of cancer and more than 500,000BC deaths predicted to have appeared in 2012 [9].
Thus, in our current research we were aim to synthesis three new indole Schiff bases derivatives as illustration in Figure1, and tested their cytotoxic activity against – AMJ 13 breast cancer cell line [10].
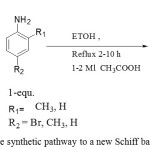 |
Figure 1: The synthetic pathway to a new Schiff bases Click here to View figure |
Experimental Section
Chemistry Part Instruments
Melting points were measured by using open capillary melting point device and the purification were taken by using Thin layer Chromatography was performed by using Silica gel sheets and the spots were observed using florescence analysis cabinet model CM-10. IR spectra were recorded on Perkin-Elmer spectrum version 1 in Diyala University, 1H, 13C NMR and APT 13C NMR spectra were recorded in DMSO on a Bruker 400 MHz spectrometer, at 400MHz for 1H NMR and at 100 MHz for 13C NMR and APT spectra in Jordan, University of science and technology, College of science, Irbid city.
Chemicals and Solvent
All the chemicals and solvents used in this research were obtained from different companies; they were used as received without further purification Such4-chlorophenyl hydrazine hydrchloride, Methyl isobutyl ketone, Dimethylformamide, and Dimethyl sulfoxide were obtained from Aldrich. Phosphoryl chloride, 4-Bromoaniline and 2,4-dimethyl aniline were obtained from Merck. Aniline and Sodium hydroxide were obtained from Thomas Baker. Glacial acetic acid was obtained from BDH. All organic solvent obtained from Scharlau. 2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-malonaldehydewas synthesized with modification of a procedure described by [8]. As shown in figure (2)
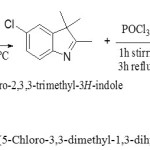 |
Figure 2: The synthetic pathway of2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene) malonaldehyde Click here to View figure |
Synthetic Methods
Synthesis of 2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-3-phenylimino-Propionaldehyde (1). As illustrated in figure 3.
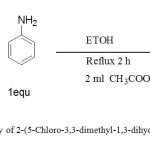 |
Figure 3: Synthetic pathway of 2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-3-phenylimino-propionaldehyde (1). Click here to View figure |
A solution of (0.75g, 3 mmol) of 2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-malonaldehyde was dissolved in ethanol 30 mL and (0.28 g, 3 mmol) of phenyl amine was dissolved in ethanol 10 mL and then added glacial acetic acid 2mL to the solution. The mixture was stirring in a water bath at 78ºC for 2h. Yellow crystals precipitate was formed, filtered off, washed with ethanol and dried in oven at 78ºC. The purity of this compound was determined by using TLC with pre-coated silica gel, which gave one spot. IR data in (cm-1): 2968 ʋ(CH aromatic), 2705 ʋ(CH aldehyde, 1672 ʋ(CH=O), 1628 ʋ(C=C), 1599 ʋ(CHN), 1402ʋ(CH3), 1230 ʋ(C-N), 820 ʋ(C-Cl) and 754 ʋ(C-H bending). 1HNMR (400MHz, DMSO, δ ppm). 13.99(s, 1H, NH), 9.42 (s,1H,HCO), 8.69(s,1H,HCN), 7.63-7.23 (8H,Ar-H), and1.60 (s,6H, 2x CH3). 13CNMR (100MHz, DMSO, δppm):188.32 (C=O), 183.62 (NH-C=C),156.76(CH=N), 149.60, 147.64, 139.51, 129.83, 129.53, 127.44, 125.45, 121.77, 119.98 and 118.17 (Ar–CH), 108.19 (O=C-C=C), 54.41(CH3CCH3), 21.64 (s, 6H, 2xCH3). APT 13CNMR shown signals for CH and CH3 appeared at negative side (below base line of the spectrum) 188.32, 156.47,129.56, 127.17, 125.18,121.50, 119.70,117.90 and 21.38 whereas quaternary carbons and carbons deuterated DMSO solvent were observed at positive side (above base line of the spectrum) 183.62, 149.33, 147.38, 139.25, 129.27, 107.92 and 54.13.
Synthesis of 3-(4-Bromo-phenylimino)-2-(5-chloro-3,3- dimethyl-1,3-dihydro-indol-2-ylidene)-Propionaldehyde (2). As shown in figure 4.
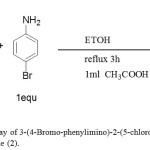 |
Figure 4: Synthetic pathway of 3-(4-Bromo-phenylimino)-2-(5-chloro-3,3- dimethyl-1,3-dihydro-indol-2-ylidene)- Propionaldehyde (2). Click here to View figure |
A solution of (0.75g, 3 mmol) of 2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-malonaldehyde was dissolved in ethanol 30 mL and (0.51 g, 3 mmol) of 4-Bromo-phenylamine was dissolved in ethanol 10 mL and then added glacial acetic acid 1 mL to the solution. The mixture was refluxed in a water bath at 78ºC for3h. A solvent was reduced to one quarter; yellow precipitate was formed, filtered off, washed with ethanol and dried in oven at 78ºC. The purity of this compound was determined by using TLC with pre-coated silica gel, which gave one spot.IR data in (cm-1): 2961 ʋ(CH aromatic), 2712 ʋ(CH aldehyde), 1665 ʋ(CH=O), 1621 ʋ(C=C), 1581 ʋ(CHN), 1394 ʋ(CH3), 1233 ʋ(C-N), 816 ʋ(C-Cl) and 769 ʋ(C-H bending). 1HNMR (400MHz, DMSO, δppm). 13.95(s, 1H, NH), 9.43(s,1H,HCO), 8.65(s,1H, HCN), 7.66-7.33(7H,Ar-H), and1.59 (s,6H, 2x CH3). 13CNMR (100MHz, DMSO, δppm):187.98 (C=O), 182.96 (NH-C=C), 156.56(CH=N), 148.92, 147.20, 139.07, 132.17, 129.21, 127.14, 121.48, 120.08, 119.53, 117.03 (Ar–CH), 108.11(O=C-C=C), 53.92(CH3 CCH3) and 21.46 (2xCH3).APT 13CNMR shown signals for CH and CH3 appeared at negative side (below base line of the spectrum) 187.87, 156.61,132.18, 127.14, 121.47, 120.17, 119.46 and 116.96 whereas quarternary carbons and carbons deuterated DMSO solvent were observed at positive side (above base line of the spectrum) 183.03.149.05, 147.26, 132.43, 129.09, 116.96, 108.08 and 53.90.
Synthesis of 2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-3-(2,4-dimethyl-phenylimino)-propionaldehyde (3). As shown in figure 5.
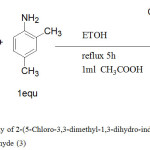 |
Figure 5: Synthetic pathway of 2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-3-(2,4-dimethyl phenylimino)-Propionaldehyde (3) Click here to View figure |
A solution of (0.7 g, 2.8 mmol) of 2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-malonaldehyde was dissolved in ethanol 25 mL and (0.33 g, 2.8 mmol) of 1,4-dimethyl aniline was dissolved in ethanol 10 mL and then added glacial acetic acid 1 mL to the solution. The mixture was refluxed in a water bath at 78ºC for5h. A solvent was reduced to one quarter; yellow precipitate was formed, filtered off, washed with ethanol and dried in oven at 78ºC. The purity of this compound was determined by using TLC with pre-coated silica gel, which gave one spot. IR data in (cm-1):-3049 ʋ(NH),2961 ʋ(CH aromatic), 2720 ʋ(CH aldehyde, 1669 ʋ(CH=O), 1625 ʋ(C=C), 1603 ʋ(CHN), 1398 ʋ(CH3), 1239 ʋ(C-N),809 ʋ(C-Cl) and780ʋ(C-H bending), 1HNMR (400MHz, DMSO, δppm). 14.09(s,1H,NH),9.40 (s,1H,HCO),8.64 (s,1H,HCN), 7.56-7.11(6H,Ar-H), 2.40 and 2.27 (s, 6H, 2x CH3) and 1.59 (s,6H, 2x CH3);13CNMR(100MHz,DMSO,δppm):187.81 (C=O), 183.60 (NH-C=C), 155.71 (CH=N), 149,45, 147.41, 135.21, 134.23, 131.42, 129.25, 127.56, 127.23, 126.86, 121.61, 119.05 and 115.09 (Ar–CH), 108.01 (O=C-C=C), 54.23 (CH3CCH3), 21.26 (2xCH3), 20.17 (o–CH3) and 17.34(p–CH3). APT 13CNMR shown signals for CH and CH3 appeared at negative side (below base line of the spectrum) 187.81, 155.66,127.21, 121.58, 119.03 and 115.13, 21.26, 20.17 and 17.34 where as quaternary carbons and carbons deuterated DMSO solvent were observed at positive side (above base line of the spectrum) 183.60, 149.43, 147.49, 135.23, 134.23, 131.40, 127.55, 126.87, 108.00 and 54.20.
Biological part
Two types of cell lines have been used in this Study. Breast cancer cell line (AMJ13) and fibroblastic and epithelial cells with normal chromosomal pictures (REF) as normal murine cell lines were used. Both of them are locally established in ICCMGR and they are maintained for use.
Determination of Solubility of Compounds Tested for in vitro Cytotoxicity
Solubility assessment of synthesized compounds was carried out according to standard test method protocol [12]. The three new compounds were dissolved in Dimethyl sulfoxide and diluted with nutrition medium RPMI-1640 to the desired concentrations (10 µg/ml, 20µg/ml , 40µg/ml and 60µg/ml). Only freshly prepared solutions were used in experiments.
Both cell lines(AMJ13 and REF) are cultured in RPMI-1640 media that contains 10% fetal bovine serum, glutamine (2 mmol/L), streptomycin (100 U/ml) and penicillin (100 U/ml), then incubated in 5% co2 at 37ºC for 24 hour. In this time the cells will grow and become monolayer. The confluent monolayer cells treated with 1 ml of trypsin/versine to provide suspension of cells, then add 10 ml of prepared media. About 200 μl of the cells were culture on clean sterile 96- well microtiter plate then let the cells for 24 hr. to make single monolayer to be ready to be treated with the our three new compounds.
Exposure day, decant the media from the cells and add 200μl from the dilutions new compounds. Each concentration was triplicate and returns the microtiter plates to the incubator. Leave wells contains only cells without treatment contains serum free media representing control cells. Three different exposure times of the cells were included in this research, 24, 48 and 72 hour. The protocol of handing and treating the cells was prepared as described by Butler, 2004 [13].
Cell Viability Assay
The cytotoxicity was determined after each exposure time using crystal violate. Decant the contents of microliter plate, add 200 μl of the crystal violate to each wells of the treated cells for 20 min. in the incubator at 37ºC. The crystal violate will stain the nuclei of the viable cell and the color will be visible to the eye. Then the plates were read by ELISA reader at 495nm. And then the inhibition rate was calculated using the following equation as recommended by [14].
%Growth Inhibition = (C–T)/C ×100 %
Were, C represent absorbance of control and T absorbance of sample.
Statistical Analysis
In this study, we used student t-test to determine the differences between the concentrations in each cell line and to determine the differences between two cells in each exposure time. The probability p was determined to be p≤0.05. Graph Pad Prism V6 was used to determine this statistical test. Excel 2010 sheet was used to draw the curves.
Results and Discussion
The new synthesized compounds were subjected to TLC; spectral studies like HNMR, 13CNMR, APT 13CNMR and FTIR, and their results are discussed below. The physical properties such as the percentage yield and melting point of the compounds (1, 2 and 3) are represented in Table No.1
Table 1: Physical properties of the synthesized compounds (1-3)
|
Compound No. |
Molecular formula |
Molecular weight |
Percentage Yield |
Melting Point ºC |
|
1 |
C19H17ClN2O |
(324.80) |
82% |
279-2810C |
|
2 |
C19H16BrClN2O |
(403.70) |
75% |
171-1730C |
|
3 |
C21H21ClN2O |
(352.86) |
84% |
169-1700C |
IR Study
The IR results of the synthesized compounds were shown absorption bands in the 4,000 – 400 cm-1 range, especially the new functional group (azomethine group CH=N) at 1599 cm-1, 1581 cm-1 and 1603 cm-1for synthesized compounds (1,2 and 3) respectively [15]. which approved to formation the accuracy chemical structure of synthesized compounds. Also strong absorption band at 1672cm-1, 1665cm-1, 1669cm-1for compounds(1, 2and 3) respectively which were belonged to (C=O)of the carbonyl group [16]. As well as stretching frequency at 1628 and 1621 cm-1forcompounds (1and2) respectively, 1625cm-1 for compounds (3) were referred to (C=C) group[17]. at the same time the synthesized compounds were appeared an absorption bands at 1230 cm-1, 1233 cm-1and 1239 cm-1 which attributed to (C-N) groups of synthesized compounds (1, 2 and 3) respectively [18].
Finally, absorption band at 1402 cm-1 and 1394 cm-1 for compounds (1 and 3) respectively and 1398 cm-1for compounds (2) were appointed to (CH3) group[19]. All these main absorption bands are approved the chemical structures of the synthesized compounds (1,2 and 3)
NMR Study
1H-NMR, 13C-NMR and APT 13C-NMR spectra were reported in DMSO (dimethyl sulfoxide) with chemical shifts in ppm and using TMS (tetramethylsilane) as standard.
1H-NMR
The 1H-NMR results for compound (3) figure (6)shown single signals at 14.09 ppm was belonged to proton of (NH) of indole ring [20].A singlet signal at 9.40ppm was referred to proton atom of carbonyl group (C=O) [21]. As well as, single signal at 8.64 ppm was attributed to proton of Schiff base group (CH=N) [22] Signals were appeared in the region between (7.56-7.11) ppm were assigned to protons of aromatic ring for (3) compound [23]. Also singlet signals at 2.40 and 2.17 ppm were assigned to two methyl groups at positions para and ortho on aromatic ring. Finally peak at 1.59 ppm was belonged to six protons of two methyl groups [24].1H NMR results of other compounds (1 and 2) are discussed and listed in table (2).
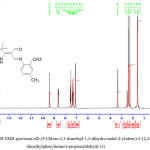 |
Figure 6: 1H NMR spectrum of2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-3-(2,4-dimethylphenylimino)-propionaldehyde (3) Click here to View figure |
Table 2: The chemical shift in ppm to1H NMR results of compounds (1-3)
|
Compound No. |
NH- |
C=O |
CH=N- |
Ar-H |
Ortho CH3 |
para CH3 |
2xCH3
|
|
1 |
13.99 |
9.42 |
8.69 |
7.63-7.23 |
– |
– |
1.60 |
|
2 |
13.95 |
9.43 |
8.65 |
7.66-7.33 |
– |
– |
1.59 |
|
3 |
14.09 |
9.40 |
8.64 |
7.56-7.11 |
2.40 |
2.17 |
1.59 |
13C NMR Study
The 13C NMR results supported 1H NMR results for compound (3) as shown on figure 7. A signal at 187. 81 ppmand at 155. 71 ppm which belonged to the carbon atom of the carbonyl group C=O and the carbon atom of the azomethine group (CH=N) respectively [25]. The signals were appear in range between (149.45, 147.41, 135.21, 134.23, 131.42, 129.25, 127.56, 127.23, 126.86, 121.61, 119.05 and 115.09 ppm) assigned to the carbon atoms of aromatic ring [26]. While the signal at108.01ppm was referred to carbon atom of (O=C-C=C) group [27], as well as a signal of carbon atom of (CH3CCH3) group was observed at 54.23. In addition, signal two groups of methyl were observed at 21. 26ppm. Finally the signals of p–CH3 and o–CH3 were appeared at 20.17 and 17.34ppm [28]. The 13C NMR results of the rest compounds (1 and 2) were given on table 3.
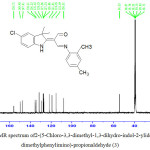 |
Figure 7: 13C NMR spectrum of2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-3-(2,4-dimethylphenylimino) propionaldehyde (3) Click here to View figure |
Table 3: The chemical shift in ppm to13C NMR results of compounds (1-3)
|
Compound No. |
C=O |
CH=N- |
Ar-CH ring |
O=C-C=C) |
CH3CCH3 |
Para CH3 |
Ortho CH3 |
2x CH3
|
|
1 |
188 |
156 |
149-118 |
108 |
54 |
– |
– |
21 |
|
2 |
187 |
156 |
148-117 |
108 |
53 |
– |
– |
21 |
|
3 |
187 |
155 |
149.-115 |
108 |
54 |
17 |
20 |
21 |
APT 13CNMR
APT 13C NMR results were further used to characterize the new three compounds. For example APT 13C NMR results of compound (3) figure8 shown signals for quaternary carbons and solvent which appeared at positive side (above of the spectrum). While CH and CH3observed at negative side (below of the spectrum). 1H, 13C NMR and APT spectrum results for the new three compounds correspond well with the expected signals and are regular with the formation of these compounds.
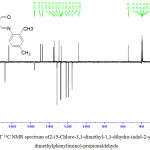 |
Figure 8: APT 13C NMR spectrum of2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-3-(2,4-dimethylphenylimino)-propionaldehyde Click here to View figure |
In Vitro Cytotoxicity Effects of Three Indole Derivatives
Cytotoxic Activity Against AMJ13 Cell Line
The synthesized of three new compounds (1, 2 and 3) were tested in vitro to determine their cytotoxicity toward breast human cancer cell line AMJ 13. Our results indicated that the cytotoxic activity of Compound (1) (figure 9) at high concentration 60µg/ml appears higher cytotoxicity against AMJ13 cells gradually increase in its cytotoxicity according to exposure time at 72hr to inhibit 70% of the cells. While values of anticancer activity were obtained with the three concentrations 10, 20 and 40 µg/ml at 72 hr. of exposure to be 60-70% as well. Further concentrations decreased, however, did not lead to the total cell death, since the cell viability was more than 90% compared to untreated cells, even at the lower concentration tested (2-10 µg/mL). Higher concentrations more than 60 µg/mL could not be prepared.
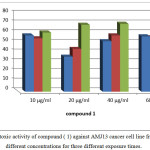 |
Figure 9: Cytotoxic activity of compound (1) against AMJ13 cancer cell line from prepared different concentrations for three different exposure times. Click here to View figure |
In case of compound (2) as presented in figure 10,the AMJ13 cells was sensitive to this derivative also at concentration 60µg/ml and the lower concentrations at 72 hr. of exposure, the inhibition of AMJ13 cells was range to be 70%. While at exposure time for 24 and 48 hr., the inhibition rate of cells were lowers than 60% for all the concentrations tested.
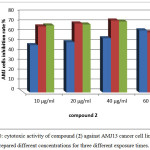 |
Figure 10: cytotoxic activity of compound (2) against AMJ13 cancer cell line from prepared different concentrations for three different exposure times. Click here to View figure |
The cytotoxic activity of compound 3 was also observed to inhibit AMJ13 cells at concentrations 60, 40 and 20 µg/ml for 48 and 72 hr. of exposures shows 70% range of cells inhibition rate. While at lower concentration 10 µg/ml also gives 60% inhibition rate at 72 hr. of exposure against AMJ 13 cells as presented in figure 11.
Totally talking about the ability of all the compounds tested and concentration 10µg/ml to be nominated against AMJ13 cell line growth at 72 hr. of exposure, choosing the lowest concentration that has the same ability to inhibit AMJ13 cells to higher ones let us to think again in using it as anticancer drug.
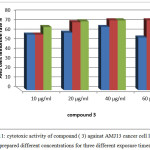 |
Figure 11: cytotoxic activity of compound ( 3) against AMJ13 cancer cell line from prepared different concentrations for three different exposure times. Click here to View figure |
Cytotoxic Activity Against REF Cell line
The prepared of three new compounds (1, 2 and 3) also tested their cytotoxic activity in vitro against REF cell line. For compound 1, all tested concentrations were appear to inhibit cell growth specially at high concentration 60 µg/ml as time dependent to give 25 % inhibition rate at 72 hr. of exposure while at lower concentrations the inhibition rates were lower means it could be safe to cell and let the REF cells grow normally specially at concentration 10 µg/ml as expressed in figure 12.
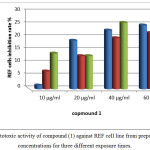 |
Figure 12: cytotoxic activity of compound (1) against REF cell line from prepared different concentrations for three different exposure times. Click here to View figure |
While REF cells when exposed to different concentrations of prepared compound (2) the cells after 24 hr. of exposure and at higher concentrations than 10µg/ml started to be very sensitive and inhibition rate was time dependent also in this case and concentration dependent. Only in concentrations 10 and 20µg/ml and at 24 hr. of exposure the cells were live normally and did not affected by this derivative to range in its inhibition from 10-20% respectively as in figure 13.
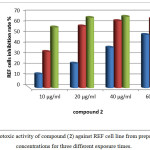 |
Figure 13: cytotoxic activity of compound (2) against REF cell line from prepared different concentrations for three different exposure times. Click here to View figure |
In compound 3, concentration 10µg/ml represented the safest concentration in the three exposure times to REF cells compared to other higher concentrations to give less than 15% inhibition rate at 72 hr. of exposure. Concentrations 20, 40 and 60 µg/ml at 24 hr. of REF cells exposure also gives lower inhibition rate 33-37% respectively as presented in figure 14.
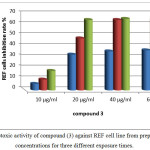 |
Figure 14: cytotoxic activity of compound (3) against REF cell line from prepared different concentrations for three different exposure times. Click here to View figure |
Reflecting from these results that compound (1) and its concentrations represent the safest to REF cell line growth did not show high inhibition rate during the exposure times. While only the lower concentration 10 µg/ml from compound (2) and (3) at 24 h of exposure give lower activity against REF cells.
Conclusions
Three new Schiff base indole derivatives [2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-3-phenylimino-propionaldehyde(1),3-(4-Bromo-phenylimino)-2-(5-chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-propionaldehyde (2),and 2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-3-(2,4-dimethyl-phenylimino)-propionaldehyde (3)] have been synthesized by reaction of 2-(5-Chloro-3,3-dimethyl-1,3-dihydro-indol-2-ylidene)-malonaldehyde with aniline derivatives , The chemical structure of the synthesized compounds have been characterized and approved by TLC, FT-IR, 1H NMR, 13C NMR and APT13C-NMR techniques. The in vitro cytotoxicity of the compound prepared against breast cancer cell line AMJ13 revealed that the lowest concentration 10 µg/ml of 1, 2 and 3 compounds has the ability to inhibit AMJ13 cells and in the same time safe to normal cell growth REF cell line. Let us to put a big highlight for further research on these compounds in cancer therapy models.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgment
The authors thank the Iraqi Center for Cancer and Medical Genetic Research, Mustansiriyah University, and Department of Chemistry, Faculty of Science, University of Diyala, Iraq, for supporting this work.
References
- Apoorva, U.;Shefali, V.;Vakacharla, S. V.;Prabha, J.;Anant. K. S.;Muralidharan, S.; Maheswaran, S.; Synthesis and characterization of 3d and 4f metal complexes of Schiff base ligands. Polyhedron.(article in press),2013, 66, 87-96.
CrossRef - Mohamed, M. I.;Hapipah, M. A.;Mahmood, A. A.;Pouya, H. Acute Toxicity and Gastro protective Effect of the Schiff Base, Ligand 1H-Indole-3-ethylene-5-nitrosalicylaldimine and Its Nickel (II) Complex on Ethanol Induced Gastric Lesions in Rats Molecules.2012, 17, 12449-12459.
- Xavier, A.;Srividhya,N.; Synthesis and Study of Schiff base Ligands. Journal of Applied Chemistry.2014,7, 2278-5736.
CrossRef - Deepa, S.;Anjani, K. T.;Sweta, S.;Gauri, S.; Pushpa, M.; Harish, C.; Anil, K. M.; Synthesis, characterization and biological activity of Schiff base analogues of indole-3-carboxaldehyde. European Journal of Medicinal Chemistry.2008,43(1),160-165.
CrossRef - Fadhil, L. F.;Metal Coordination Behavior And Biological Activities of Indole And Quinazoline Derivatives. PhD thesis, Department Of Chemistry. Faculty of Science. University of Malaya.2015.
- Manish, R.;Kumanan, R.;Duganath, N.;Srinivasa,; M. M.;Nazeer, A.;Subramanyam, S.; Synthesis, Characterization and Pharmacological Screening of 2-methyl-1H Indole-3-Carboxylic Acid [2-(2-Substituted-Phenyl)-4-Oxo-Thiazolidin-3-Yl]-Amides Derivatives. International Journal of Chemical Sciences and Applications.2011,2(1),91-99.
- Smith, R.;A.;Cokkinides,V.;Eschenbach, A.C.; American Cancer Society guidelines for the early detection of cancer. cancer journal for clinicians.2002,52,8-22.
- Xiong, L., Liang, B.;Bai, M.;Liu, J.; Impact of interaction between PPAR alpha and PPAR gamma on Breast cancer risk in Chinese Han population. Clinical Breast Cancer.2016, 17, 336–340.
- Alejandro, D. S.; Graciela, A.; David, F.;Monica, S. S.; Female Breast cancer in Central and South America. Cancer Epidemiology.2016, 44, 110-120.
CrossRef - Al-Shammari, A.; Alshami, M.; Urman, M.; Al-mukhtar, A.; Yaseen, N.; Raad, K. and Hussien,A. Establihment and characterization of a receptor-negative, hormone-nonresponsive breast cancer cell line from Iraqi patient. Breast Cancer (Dove Med Press), 2015, 7, 223-230.
CrossRef - Baradarani, M.; Afghan, A.; Zebarjadi, F.; Hasanzadeh, K.; Joule, J.; The synthesis of 3,3-dimethyl-2-(1-aryl-1h-pyrazol-4-yl)-3h-indoles. Journal of Heterocyclic Chemistry.2006, 43, 1591-1595.
CrossRef - The National Toxicology Program (NTP) Interagency Center for the Evaluation of & Alternative Toxicological Methods (NICEATM): Test Method Protocol for Solubility Determination Phase III.2003. 1-10.
- Butler, M.; Animal cell culture and technology. The basics. Oxford university press. New York,book.2004. 2, 1-256.
CrossRef - Freshney, R. I.; Culture of animal cells. A manual for basic technique. Fifth ed. Willey liss. A Juhn wiley and sons. Inc Pup. New York, book.2005,1- 672.
- Ngan,N. K.; Lo, K.M.;Synthesis, structure studies and electrochemistry of molybdenum (VI) Schiff base complexes in the presence of different donor solvent molecules. Polyhedron.2011, 30,22-32.
CrossRef - El-Refaie, K.; Mohamed, A.; Khalil, S.; Ahmed, E.; Preparation of organophilic montmorillonite-based dimethyl amino benzaldehyde-Schiff-base as antibacterial agents. .Arabian Journal of Chemistry. 2016, 9, 574-585.
- Montazerozohori, M.;Musavi, M.; Synthesis, spectral, crystal structural, antimicrobial, DNA interaction and thermal behavior of some new zinc halide complexes:3Dsupramolecular structure of zinc bromide complex. Arabian Journal of Chemistry. 2014, 7, 656-667.
- Rayees, A.S.;Mohmmad, Y.W.; Sheikh,S.A.;Adil, H.; Synthesis, characterization and biological screening of some Schiff base macro cyclic ligand based transition metal complexes as antifungal agents. Arabian Journal of Chemistry.2016, 9, 743-751.
CrossRef - Razieh, M. A.; Mehdi, M.B.;Arash, A.; Synthesis of new heterocyclic compounds using 2-(4,7-dichloro-3,3-dimethylindolin-2-ylidene)malonaldehyde.CurrentChemistry Letters.2013, 2,13–20.
- Faraj,F.L.;Khaledi, H.;Morimoto, Y.A.;TetradentateDiiminato Ligand Containing Phenolate Substituents: Flexivalent Coordination to MnIII, CoIII, NiII, and CuII. European Journal of Inorganic Chemistry.2014, 33, 5752-5759.
CrossRef - Misra, U.; Hitkari, A.; Saxena, A.; Gurtu, S.;Shanker, K.; Biologically active indolylmethyl-1,3,4-oxadiazoles,1,3,4-thiadiazoles,4H-1,3,4-triazoles and 1,2,4-triazines.Eurpean Journal of Medicinal Chemistry. 1996, 31, 629-634.
CrossRef - Suzan, A.M.;Wamidh, H.T.;Mohammad, S.;Mohammad,S.;Murad, A.;Synthesis, characterization, and antimicrobial activity of Schiff bases derived from benzaldehydes and 3,30-diaminodipropylamine.Arabian Journal of Chemistry.2015, 8,850–857.
CrossRef - Zahedifard, M.;Faraj, F.; Paydar, M.;Synthesis, characterization and apoptotic activity of quinazolinone Schiff base derivatives toward MCF-7 cells via intrinsic and extrinsic apoptosis pathways. Scientific reports.2015,5, 497–514.
CrossRef - Alamgir, M.;Synthesis and reactivity of some activated heterocyclic compounds. Ph.D. Thesis, The University of New South Wales Sydney, Australia,2007.
- Ngui, K.N.; Kong, M.L.;CheeSeng, R.W.; Synthesis, structure studies and electrochemistry of molybdenum(VI) Schiff base complexes in the presence of different donor solvent molecules.Polyhedron.2011, 30,2922–2932.
CrossRef - Muhammad, A.;Karamat, M.;Synthesis, Characterization and Biological Activity of Schiff bases..International Conference on Chemistry and Chemical Process.2011, 10, 556-564.
- Fadhil, L. F.; Hamid, K.;Hamed, K.; Hapipah, M. A.; Indole-Fused Epoxy-1,5-Diazocinesas Cancer-Selective Cytotoxic Agents. Heterocyclic Chemistry, 2017, 54, 2071–2074.
CrossRef - Sami, S.;Hooshang, V.;Abdolhossien, M.; Omid, L.; New 3H-Indole Synthesis by Fischer’s Method. Part I. Molecules.2010, 15, 2491-2498.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









