Equilibrium Studies of Ternary Complexes of Cu(II) with Venlafaxine hydrochloride Drug and Some Amino Acids
Department of Chemistry, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Corresponding Author E-mail: wwww_09@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340170
The various complexes stability constants (SCs) of Cu (II) with venlafaxine HCl (V) has been determined using a potentiometric titration technique as primary ligand. While the secondary ligands was symbolized by (L) referring to selected amino acids, the two were under experimental conditions (25°C and ionic strength 0.1 molL NaNO3). The complexes containing mixed ligand ternary were occurring with each other generally in most cases at high pH with binary complexes progressively deviation of particular metal complexes. Comparative stabilities of (TC) were equated with those of the corresponding binary complexes in terms of Δ log
KEYWORDS:Stability Constant; Venlafaxine HCl ; Amino Acids; Potentiometric Titration
Download this article as:| Copy the following to cite this article: Alturiqi A. S. Equilibrium Studies of Ternary Complexes of Cu(II) with Venlafaxine hydrochloride Drug and Some Amino Acids. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Alturiqi A. S. Equilibrium Studies of Ternary Complexes of Cu(II) with Venlafaxine hydrochloride Drug and Some Amino Acids. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=41623 |
Introduction
The metal ions interaction with its ligand plays vital role in biological systems. To understand the complexes physiological process and the drugs mode of action as well as their effects on different body systems the knowledge of metal complexes with drugs was necessary. Metal complexes formation depends on metal ligand selectivity in complex media.
It was important to metal ligand selectivity measurement determine the stability constant (SC) of metal complexes with drugs expressing relative strength of metal ligand bonds 1. It was found that metal complexes of drugs were more potent than drugs 2. It acting a vital role in transportation, detoxification and catalytic process.
Venlafaxine hydrochloride, (1-[2-dimethylamino)-1-(4-methoxy phenyl) ethyl] cyclohexanol) hydrochloride structurally represent a third-generation, novel phenethyl bicyclic antidepressant3. Both serotonin and noradrenalin may inhibit synaptosomal re-uptake by Venlafaxine, but it shows a moderately weak dopamine re-uptake 4 inhibitor. The venlafaxine hydrochloride chemical structure was depicted in Fig.1.
A literature survey showed that several analytical methods have been stated to venlafaxine determination in its pure form, pharmaceutical preparations and biological fluids 5 -14.
In the present investigation, the binary formation and Cu (II) (TC) with venlafaxine HCl (V) and pH-metric technique was utilized to investigate some amino acids (glycine, alanine, serine, ornithine and methionine). Complexes constants formation were determined at 25°C and ionic strength 0.1 M NaNO3. The relative stabilities of (TC) were compared to those of corresponding binary complexes expressing in Δ log and % RS values. Then complexes concentration distribution were assessed.
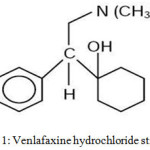 |
Figure 1: Venlafaxine hydrochloride structure. Click here to View figure |
Experimental
All chemicals grade were of analytical, and throughout the experiments binary distilled water was used. Venlafaxine hydrochloride, glycine, alanine, serine, methionine, ornithine, methylamine. HCl and imidazole were purchased from Sigma Chemical Co. and utilized without extra purification. Cu (NO3)2·2H2O and NaNO3 were delivered by BDH-Biochemicals Ltd Poole. NaOH free from carbonate (titrant) was equipped and standardized against potassium hydrogen phthalate solution. In deionized H2O all solutions were equipped.
Griffin pH J-300-010 G Digital pH meter at 25±0.1°C in a double-walled glass vessel conditions was used to perform potentiometric titrations. Before pH measurements the electrode with standard buffer solutions was calibrated (pH4.0 and 10.0). For each titration a NaNO3 solution was used to kept ionic strength constant (0.10 mol/L), with 40 cm-3 as a total volume.
The succeeding solutions were primed and titrated potentiometrically in contradiction of standard carbonate- free NaOH (0.1 mol/L) solution: (a) 40 cm-3 of a solution containing 1.25×10−3 mol/L of (V or L) + 0.1 mol/L NaNO3. (b) 40 cm-3 of a solution containing 1.25×10−3 mol/L Cu (II) + 2.5× 10−3 mol/L V or L + 0.1 mol/L NaNO3. (d). 40 cm-3 of a solution containing 1.25×10−3 mol/L Cu (II) + 1.25×10−3 mol/L V + 1.25×10−3 mol/L L + 0.1 mol/L NaNO3. Double-distilled water had been added to adjust total volume at 40 cm-3 in each case. Titration of mixture (a) to evaluate the protonation constants of V (or L). However titration of mixture (b) had been used to evaluate the Cu (II) – V and Cu (II)-L complexes constants formation. As though, Cu (II) – V-L stability constants for (TC) were estimated by mixture (c) titration.
All of titrations has been needed to adding HNO3 solution, so that at the beginning of the titrations they were fully protonated. A pKw value of 13.87±0.05 at 25°C was used to estimate [OH] values.
HYPERQUAD15 was the computer program which utilized to Calculations accomplished. The created complexes stoichiometric and its SCs were determined by testing a various potential alignment models for studied systems. Diagrams of species distribution were gotten by SPECIES 16 program.
Results and Discussion
In present study, the protonation constants of all ligands which determined were scheduled in Table 1. It was showed that all amino acids protonation constants identified in this survey were very close to earlier reported values for those ligands (Table 1) 17, 18. Venlafaxine pKa value appear as 9.6, it predominantly means that present in ionized form at a physiologic pH.
Binary Copper (II) Complex Formation Equilibria with Venlafaxine
The formation constants of Cu (II) binary complexes with its venlafaxine had been determined by fitting basis potentiometric data on the possible composition models. The selected model with the best statistical fit was found to consist of 110 and 120 complexes. SCs values of their complexes were assumed in Table (1). Venlafaxine Potentiometric titration curves shown in Fig 2 in presence and absence of Cu (II) ion. Displacements of the curves have invariably been gained relative to that of free venlafaxine curve, indicating release of protons associated with complex formation. SCs of these complexes were calculated (according to (3) and (4)).
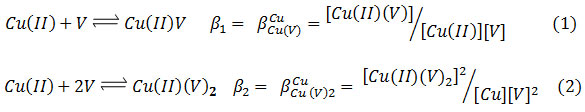
The Cu (II)-V system diagram of concentration distribution is given in Fig. 3. With increasing the pH value the concentration of Cu (II)-V (110) species increase. Also when attains a maximum concentration of 98.0% at pH 6.8. Further pH increasing is accompanied by a decrease in Cu (II)-V species concentration as well as an increase in Cu (II)- (V)2 species concentration.
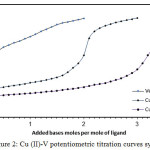 |
Figure 2: Cu (II)-V potentiometric titration curves system. Click here to View figure |
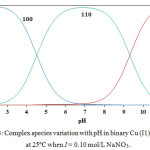 |
Figure 3: Complex species variation with pH in binary Cu (I1) – V systems at 25ºC when I = 0.10 mol/L NaNO3. Click here to View figure |
Table 1: Ligands constants and stability constants of Cu (II) complexes in binary systems at 25C° and 0.1 mol/L ionic strength.
| System | l | p | qa | log βb |
| 1 | 0 | -1 | -7.44(0.03) | |
| [Cu(H2O)4]2+ | 1 | 0 | -2 | 15.09(0.02) |
| Venlafaxine (V) | 0 | 1 | 1 | 9.60 (0.02) |
| 1 | 1 | 0 | 8.26 (0.02) | |
| 1 | 2 | 0 | 10.36 (0.01) | |
| 1 | 1 | -1 | -2.55(0.04) | |
| 1 | 1 | -2 | 9.64(0.03) | |
| System | l | p | qa | log βb |
| Glycine | 0 | 1 | 1 | 9.62(0.02) |
| 0 | 1 | 2 | 11.90(0.01) | |
| 1 | 1 | 0 | 8.11(0.02) | |
| 1 | 2 | 0 | 15.07(0.03) | |
| Methylamine | 0 | 1 | 1 | 10.55(0.01) |
| 1 | 1 | 0 | 6.01 (0.01) | |
| 1 | 2 | 0 | 11.34 (0.03) | |
| Imidazole | 0 | 1 | 1 | 7.04(0.01) |
| 1 | 1 | 0 | 4.50 (0.01) | |
| 1 | 2 | 0 | 7.66 (0.05) | |
| Alanine | 0 | 1 | 1 | 9.69(0.01) |
| 0 | 1 | 2 | 11.88(0.02) | |
| 1 | 1 | 0 | 8.01(0.03) | |
| 1 | 2 | 0 | 14.48(0.01) | |
| Serine | 0 | 1 | 1 | 9.11(0.01) |
| 0 | 1 | 2 | 11.46(0.01) | |
| 1 | 1 | 0 | 8.64(0.03) | |
| 1 | 2 | 0 | 16.43(0.03) | |
| Methionine | 0 | 1 | 1 | 9.06(0.01) |
| 0 | 1 | 2 | 11.32(0.04) | |
| 1 | 1 | 0 | 8.51(0.03 ) | |
| 1 | 2 | 0 | 16.05(0.03) | |
| Ornithine | 0 | 1 | 1 | 10.58(0.00) |
| 0 | 1 | 2 | 19.43(0.02) | |
| 0 | 1 | 3 | 21.39(0.02) | |
| 1 | 1 | 0 | 10.97(0.05) | |
| 1 | 2 | 0 | 16.23(0.06) |
a) L, p and q values represent stoichiometric coefficients corresponding to Cu (II), V or amino acids and H +, respectively.
b) Standard deviations were given in parentheses.
Ternary Copper (II) Complex Formation Equilibria Comprising Venlafaxine and Some Amino Acids
The SCs –complex formation between Cu (II), V, and L is represented by the general equilibria (charges briefly omitted):
![]()
Formed species Stability was measured with stoichiometric equilibrium constant βlpqr expressed where ionic strength, temperature and concentrations were constant.
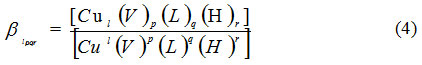
Where Cu (II) ion numbers described by l, p, q and r for venlafaxine (V), amino acids (L) and proton, respectively, in the complex [(Cu) l (V)p (L)q (H)r].
SCs
![]()
and
![]()
were compared with each other in order to decide which one of the ligands was responses to mixed ligand complexes formation, besides which one acting as a primary or secondary ligand. For this supposition, the succeeding equations were utilized:
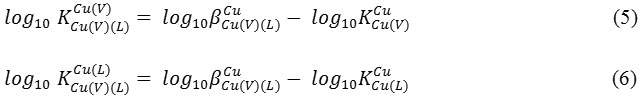
![]()
and
![]()
were calculated as shown in |(Table 2) for each mixed ligand system. Venlafaxine drug doings as a primary ligand particularly in the Cu (II): V: glycine and Cu (II): V: alanine systems and amino acids act as a primary ligand in Cu (II): V: serine, Cu (II): V: methionine and Cu (II): V: ornithine systems.
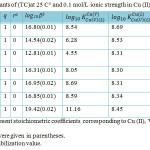 |
Table 2: Formation constants of (TC)at 25 C° and 0.1 mol/L ionic strength in Cu (II)-V- amino acids systems. Click here to View table |
When the titration results for the Cu (II)-V-amino acids analyzed it shows that 1:1:1 complexes formation with SCs higher than those of the corresponding monodentate methylamine and imidazole complexes. We can state that amino acids may bind via both groups amino and carboxylate.
TC Stability that glycine inclusive were higher than those holding alanine. It is suggested that the steric hindrance, produced by the presence of a methyl group on the carbon bearing amino group (alanine), is responsible for that lower ternary complexes stability.
The mixed-ligand complexes SCs of serine and methionine were in fair covenant with those of other studied amino acids, when the difference in acid dissociation constants of amino acids was considered. So in mixed-ligand complex formation, serine and methionine participate as a substituted glycinates. Consequently, the methionine thioether group and serine β-alcoholato-group do not participate in complex formation, as previously reported for copper (II) complexes19, 20.
Ornithine complex SC was higher than those of a-amino acids. This may be elucidate that ornithine in all probability chelates through both amino groups.
Estimation of various complex species in its concentration distribution was useful elucidation for the complexes concentration as a pH function. In all investigated systems, the ternary complex concentration increases with a pH increasing. With assorted amino acids investigated, the Cu (II)-V-amino acids concentration was ranged from almost 42% to 88% with 8.0 -9.8 pH range. In these systems, to illustrate the main observed features in species distribution plots, the achieved speciation diagram for Cu-V-serine systems, which occupied as a representative, is shown in Fig. 4.
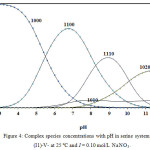 |
Figure 4: Complex species concentrations with pH in serine systems Cu (I1)-V- at 25 ºC and I = 0.10 mol/L NaNO3. Click here to View figure |
Both ternary and binary complexes, relative stability can be quantitatively expressed in a number of different ways 21. It has been established that comparison and it could be better as a ∆ log10 K, which defined by Eq. (8):
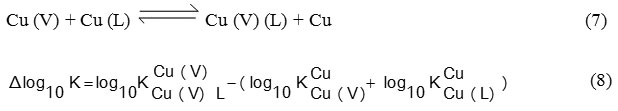
In this paper, Δ log10 K values for studied (TC) were positive and listed in (Table 2). It means that amino acids form more stable complexes with Cu (V) than with the free Cu (II) ion. This may be attributed to many factors, stacking of intramolecular aromatic-ring, hydrogen bond, and a π–π cooperative effect between ligands or others.
Another parameters may be that percent relative stabilization (% RS) for quantifying ternary complex stability, which defined as 22:
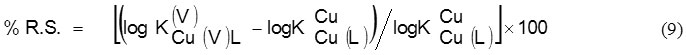
The % R.S. values have been calculated (Table 2). % R.S. is positive for all systems. For enhanced stabilities occurrence it may be considered as an evidence. Moreover positive values of % R.S. were of one accord with ∆ log K values.
References
- Thomas, G. Medicinal Chemistry, John Wiley and Son Co. Ltd. London (2002).
- Sarkar, B. Medicinal Inorganic Chemistry Rev. Med., 41, 2535-2544 (1999).
- Clarke’s, Analysis of Drugs and Poisons, Third edition, Edited by Moffat A C,Osselton M D, Widdop B, Pharmaceutical Press, 2, 2004, 1694- 1995.
- Goodman& Gilman’s, The Pharmacological Basis of Therapeutics, 11th edition, Mc Graw Hill Medical Publishing Division, London, 2005, 447-485
- Salgado-Petinal, C.; Lamas, J.P.; Garcia-Jares, C.; Iompart, M. L.; Cela, R. Anal. Bioanal. Chem. 2005, 382(6), 1351–1359.
CrossRef - Wille, S. M. R.; Maudens, K. E,; Van Peteghem, C. H.; Lambert, W.E.E. J. Chromatogr. A. 2007, 1176, 236-245.
CrossRef - Mandrioli, R.; Mercolini L, Cesta R, Fanali S. J. Chromatogr. B, 2007, 856, 88-94.
CrossRef - Nageswara Rao, R.; Narasa Raju, A. J. Sep. Sci., 2006, 29(18), 2733- 44.
CrossRef - Duverneuil, C.; de la Grandmaison, G. L.; Mazancourt de, P.; Alvarez, J. C. Ther. Drug Monit. 2003, 25, 565-73.
- Lima, J. L.; Loo, D. V.; Delerue-Matos, C.; da Silva, A. S.; Farmaco. 1999 ,31; 54(3),145-8.
- Wei, Z.; Bing-Ren, X.; Cai-Yun, W. Biomed. Chromatogr. 2007, 21, 266-72.
CrossRef - Raut, B. B.; Kolte, B. L.; Deo, A. A.; Bagool, M . A.; Shinde, D. B. J. Liq. Chromatogr. Relat. Technol. 2003, 26(8), 1297-1313.
CrossRef - Titier K, Castaing N, Scotto-Gomez E, Pehourcq F, Moore N, Molimard M.Ther. Drug. Mon. 2003, 25(5), 581- 587.
CrossRef - Rudaz, S.; Stella, C.; Balant-Gorgia, A. E.; Fanali, S.; Veuthey, J. L. J Pharm Biomed Anal. 2000, 1, 23(1):107-15.
- Gans, P.; Sabatini, A.; Vacca, A. Talant 1996, 43, 1739–1753.
CrossRef - Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Coordination Chemistry Reviews 1999, 184, 311–318.
CrossRef - Ammar, R .A.; Al-Mutiri, E.; Abdalla, M. A. J. Solution Chem. 2010, 39, 727–737
CrossRef - Azza, A. S.; Mahmoud, M. A. M.; Mohamed, M. S.; J. Solution Chem. 2006, 35, 853–868.
CrossRef - Pettit, L.; Swash, J. J. Chem. Soc. Dalton Trans. 1976, 2416.
CrossRef - Kozlowski, H. Becock, B. R.; Delarnlle, J. L.; Loucleux, C. ; Ancian, B. Inorg. Chim. Acta 1983, 78, 31.
- Zhang, F.; Liu, Q. J. Coord. Chem. 1993. 28, 197–202 .
CrossRef - Khalil, M. M.; Radalla, A. M. Talanta 1998, 46: 53-61.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.










