Influence of Ball Milling on CdO Nanoparticles Prepared by Thermal Decomposition
Sagi Rani C1 , Thangaraj G2, Suresh D. M2 and Joseph John N3
, Thangaraj G2, Suresh D. M2 and Joseph John N3
1Department of Physics, Noorul Islam centre for Higher Education, Noorul Islam University, Kumaracoil, Tamil Nadu - 629180, India.
2Department of Physics, Sethupathy Government Arts College Ramanathapuram, Tamil Nadu - 625 502., India.
3Department of Physics, Government Arts College, Udhagamandalam, Tamil Nadu - 643 002, India.
Corresponding Author E-mail: sagirani.c@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340167
Cadmium oxide has found its applications in various areas of research such as solar cells, phototransistors, photodiodes, transparent electrodes and gas sensors. In the present work CdO nano particles were successfully synthesized from cadmium acetate through thermal decomposition at high temperatures by adopting the solid state reaction. The prepared nano particles are subjected to ball milling for different timings. From X-ray diffraction it was found that the particle size decreases for an increase in the time of ball milling. The structure and the elemental composition were analyzed using SEM with EDAX. The optical band gap of CdO nano particles were determined using UV-Vis absorption spectroscopy. The thermal properties were determined using TGA/DTA analysis.
KEYWORDS:Nanoparticle; Ball Milling; XRD; PL; TGA/DTA
Download this article as:| Copy the following to cite this article: Sagi R. C, Thangaraj G, Suresh D. M, Joseph J. N. Influence of Ball Milling on CdO Nanoparticles Prepared by Thermal Decomposition. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Sagi R. C, Thangaraj G, Suresh D. M, Joseph J. N. Influence of Ball Milling on CdO Nanoparticles Prepared by Thermal Decomposition. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42340 |
Introduction
Nano technology has shrunken down the various versions of modern day particles through the creation of nano particles. [1-2]. Type II-VI semiconductors, as a class of materials, have been and still are the subject of much intensive investigation. The growth of semiconductor technology in the early 1950’s highlighted the limitations of silicon and germanium, which perhaps the character and the magnitude of the forbidden energy gap were the most disadvantageous. At first the extension in the range of energy gaps was sought in these III-V semiconductors, where considerable success has been achieved with InSb and GaAs in the low and high energy gap areas respectively. GaAs is today probably the most developed and well understood compound in existence. Concurrently with the later developments in the III-V semiconductors, systematic studies were made for several of these compounds. The results of these studies have revealed much about the general nature of the II-VI semiconductors and the feature of chemical stability of the higher energy gap materials at room temperature offers an immediate advantage over the unstable III-V phosphides. Type II-VI semiconductors, in their broadest sense, include compounds formed from elements of group II and group VI of the periodic table [3]. Generally Cadmium oxide (CdO) is a II-VI n-type semiconductor and has interesting properties like large band gap, high electrical conductivity and high transmission in the visible region. [4].
CdO has various properties like optical, electrical and catalytic properties that can be used to photo degrade some of the organic compounds, dyes pigments and of environmental pollutants. [5-7]. Due to its low resistivity and high carrier concentration, it has gained most of its interest towards opto electronic devices. [8-10]. Bulk CdO is an n-type semiconductor with a direct band gap of 2.7 eV. [11]. Due to its specific electrical and optical properties it has its applications in photovoltaic cells, solar cells, photo transistors , IR reflectors, gas sensors and also in a variety of other materials.[12-16]. A reduction in particle size can strongly influence the optical and electronic properties of nanomaterials [17]. These properties are dependent on the microstructural characteristics and chemical composition that can be controlled during the synthesis process [18,19]. In this paper we report the synthesis and characterization of CdO via thermal decomposition. The results obtained are reported herewith.
Materials and Methods
Analytical reagent (AR) grade cadmium acetate purchased from Merk India was used for the synthesis of CdO nanoparticles by solid state reaction. The chemicals used in the present study were used as such without any further purification.
For the synthesis of CdO nanoparticles required quantities of cadmium acetate dehydrate is taken in an alumina crucible and is subjected to decompose thermally in its dry media thus employing the technique of solid state reaction. The thermal decomposition was carried out in a muffle furnace 9500C for a period of 24 hours. As a result brown coloured CdO nano particles were obtained. These CdO nano particles thus obtained were subjected to ball milling for a period of 5 hrs and 10 hrs respectively.
Results and Discussion
The CdO nano particles thus prepared were characterized by various techniques like XRD, FTIR, PL, and TGA/DTA.
XRD Analysis
The powder XRD measurements were carried out by a XPERT-PRO diffractometer with monochromatized Cu K-Alpha radiation [K-Alpha= 1.54443Å]. Fig: 1. shows the XRD pattern for the prepared CdO nano particles, for the as prepared, ball milling for 5 hrs and ball milling for 10 hrs respectively. The sharp and well defined peaks indicate the crystalline nature of CdO. The XRD pattern has confirmed the formation of CdO nano particle (JCPDS card no:75-0594) that exhibits various peaks at 32.850, 37.90, and 55.930 and they can be readily indexed as (111), (200) and (220) respectively and correspond to the cubic nature of CdO. No other impurity peaks were detected indicationg that the obtained CdO was phase pure.
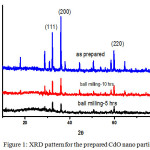 |
Figure 1: XRD pattern for the prepared CdO nano particles Click here to View figure |
The average particle size is estimated using the Debye-Scherrer formula
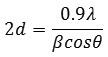
Where, β is the Full Width at Half Maximum intensity [FWHM] corresponding to the diffraction angle 2θ in radian, and λ is the wavelength of Cu-kα radiation.[20,21]. The average particle size for the prepared nano particles is found to be 94.42 nm, 37.23 nm and 7.59 nm for the as prepared, ball milled for 5 hrs and ball milled for 10 hrs respectively. Thus it is found that the particle size of the prepared nano particle decreases with the increase in the time of ball milling.
FTIR Analysis
Fig: 2 shows the FTIR spectra for the prepared CdO nano particle, which was analyzed from 4000 to 400 cm-1.
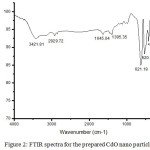 |
Figure 2: FTIR spectra for the prepared CdO nano particle Click here to View figure |
The broad bands at 3421.81 cm-1 and 2929.72 cm-1 are due to the vibrations of OH group indicating the presence of small amount of water molecules adsorbed on its surface. The peaks around 520.74 cm-1 and 420.302 cm-1 is assigned to Cd–O of CdO, confirming the formation of the pure CdO nanoparticles[22].
PL studies
Photoluminescence spectra for the prepared CdO nano particles is recorded using Spectroflurometer, under 450 high pressure and is shown in fig: 3. Xenon lamp is used as an excitation source. The Photoluminescence (PL) spectrum of the prepared CdO nano particles shows a sharp emission peak at 360.99 nm. The band gap energy at this emission peak is found to be 3.44 eV, using the formulax
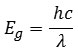
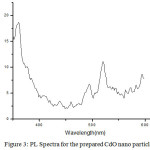 |
Figure 3: PL Spectra for the prepared CdO nano particle Click here to View figure |
TGA/DTA Analysis
The TGA-DTG curves were used to determine the appropriate starting temperature for the calcinations process. Fig: 4 represent the TGA/DTA curve for the prepared CdO nano particle. Thermal Gravimetric analysis (TGA) and Differential Thermal Analysis (DTA) were carried out between 00C and 12000C and it is clear that an exothermic DTA peak is recorded which indicates the formation of CdO phase through oxidation decomposition process. The TGA curve shows an apparent weight loss in different stages. The first decomposition upto 980C is due the adsorbed water molecules
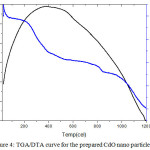 |
Figure 4: TGA/DTA curve for the prepared CdO nano particle Click here to View figure |
Conclusions
In the present study CdO nano particles were successfully prepared by the method of solid state reaction from Cadmium acetate dihydrate. The prepared nano particles are characterized by adopting various techniques like XRD, PL, and TGA/DTA. From XRD it was found that the particle size of the prepared nano particles increases with the increase in the rate of ball milling. The various spectral vibrations of the sample were analyzed from the FTIR characterization. The thermal stability of the sample is obtained from TGA/DTA analysis. The optical band gap for the prepared nano-composites is found as 3.44eV.
References
- Durga Vijay Karthik, D.; Kirithika, M.; Prithivikumaran, N.; Jeyakumaran, N. Int.J.Nano Dimens. 2014, 5(6), 557-562.
- Sagirani, C.; Athira, P.; Joseph John, N. , Nanosystems : Physics chemistry Mathematics. 2016, 7 (4), 0-2.
- Ajin Sundar, S.; Joseph John, N.; Nanosystems : Physics chemistry Mathematics. 2016, 7 (6), 1-7
- Ajin Sundar, S.; Joseph John, N. Int. J. Eng. and Applied Sciences. 2016, 3(3), 26-29.
- Lide, D.R.; CRC Handbook of chemistry and physics. 1996, 77th edition.
- Karunakaran, C.; Dhanalakshmi, R. Centr.Eur J.Chem. 2009, (7), 134-137.
- Karunakaran, C.; Dhanalakshmi, R.; Gomathisankar, P.; Manikandan, G. J. Hazard Mater. 2010, (176), 799-806
CrossRef - Nezamzadeh-Ejhieh, A.; Banan, Z. Desalination, 2011, (279), 146-151.
CrossRef - Serbetci, Z.; Gunduz, B.; Al-Ghamdi, A. A.; Al-Hazmic, F.; Arik, K.; EL-Tantawy, F.; Yakuphanoglu, F.; Farooq, W. A. Acta Physica Polonica. 2014, (126), 798-807.
CrossRef - Yan, M.; Lane, M.; Kannewurf, C. R.; Chang, R. P. H. Appl.Phys.Lett. 2001, 78, 2342.
CrossRef - Zheng, B. J.; Lian, J. S.; Zhao, L.; Jiang, Q. Vacuum. 2011, 85, 861.
CrossRef - Deepa, G.; Mahadevan, C. K., IOSR-JAP 2013, 5,15-18.
- Barve, A. K.; Gadegone, S. M.; Lanjewar, M. R.; Lanjewar, R. B. IJRITCC. 1985, 63, 2806-2010.
- Champness, C. H.; Ghoneim, K.; Chencan,. J. K. J.Phys. 1985, 63,767.
- Su, L. M.; Grote, N.; Schmitt, F. Electron let. 1985, 20, 716.
- Darezereshki, E.; Bakhtiari, F. Journal. Mining metallurgy. 2013,49(1), 21.
CrossRef - Sharma T. P.; DineshPatidar ; Saxena N. S.; KananbalaSharma ; Indian J. Pure Appl.Phys. 2006, 44, 125–128.
- Zhang L.; Wang L.; Jiang Z.; Xie Z.; NanoscaleRes.Lett. 2012, 7, 312.
CrossRef - Sato R. ; Kanehara M.; Teranishi T. ; Small 2011, 7, 469–473.
CrossRef - Cullity, B. D. Elements of X-Ray Diffraction. 1978, 2nd Ed.
- Ajin Sundar, S.; Joseph John, N. Int. J. Eng. and Applied Sciences. 2016, 3, 26-29.
- Gholam Reza Khayati,; Hamid Dalvand,; Esmaeel Darezereshki,; Ahmad Iranneajad,. Materials letters. 2014, 115, 272-274.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









