HMX Synthesis by using RFNA/P2O5 as a Novel Nitrolysis System
Khadijeh Didehban1, Mohammad Ali Zarei1, Morteza Radfar2 and Yadollah Bayat2
1Department of Chemistry, Payame Noor University, P.O. Box 19395-3697, Tehran, Iran.
2Department of Chemistry, Malek Ashtar University of Technology, P.O. Box 16765-3454, Tehran, Iran.
Corresponding Author E-mail: ma.zarei51@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340169
A novel and efficient approach for the synthesis of octahydro- 1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) was achieved through nitrolysis of 1,3,5,7-tetracetyl-1,3,5,7-tetraazacyclooctane (TAT) in the presence of red fuming nitric acid (RFNA) and P2O5 . various parameters such as temperature, concentration of nitrating agent, time and mole ratio of RFNA/P2O5 has been investigated to develop the optimum conditions. The good yields, high purities, easy work-up and short reaction times are advantages of this method.
KEYWORDS:HMX Tetraazacyclooctane; RFNA
Download this article as:| Copy the following to cite this article: Didehban K, Zarei M. A, Radfar M, Bayat Y. HMX Synthesis by using RFNA/P2O5 as a Novel Nitrolysis System. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Didehban K, Zarei M. A, Radfar M, Bayat Y. HMX Synthesis by using RFNA/P2O5 as a Novel Nitrolysis System. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=41698 |
Introduction
Nitrolysis is one of the key processes in synthetic chemistry and widely used in industry, nitrolysis process mostly requires concentrated amounts of nitric acid, great number of the energetic materials such as HMX are produced by various nitrolysis processes[1-5]. HMX (1,3,5,7-Tetranitro-1,3,5,7-tetraazacyclooctane), is one of the most powerful high-melting explosives which is useful for military and non-military applications[6,7]. In the past decades various methods for HMX prepration have been reported in the literature, one of the first methods developed by Bachmann in 1951[8] but there are several undesirable features including slow rate of production, poor yield, large amount of hazardous wastes and great amount of by-products such as RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) and mixture of cyclic and linear nitramines.
In order to improve HMX production other synthetic approaches involving TAT (1,3,5,7-tetracetyl-1,3,5,7-tetrazacyclooctane), DADN(1,5-diacetyl-3,7-dinitro-1,3,5,7-tetrazacyclooctane) or DANNO (1,5-diacetyl-3-nitro-5-nitroso-1,3,5,7-tetrazacyclooctane) have been proposed, the main precursor for these alternative routes is DAPT (3,7-diacetyl-1,3,5,7-tetraazabicyclo-[3.3.1]-nonane) which could be easily prepared from hexamine and acetic anhydride in good yield.
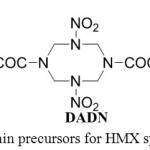 |
Figure 1: Main precursors for HMX synthesis Click here to View figure |
According to Siele’s works [9] DADN gave a better yield in compare to the Bachmann procedure but this synthesis involves use of sulfuric acid and polyphosphoric acid which are not required in the Bachmann process.
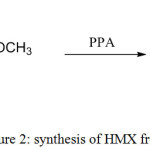 |
Figure 2: Synthesis of HMX from DADN Click here to View figure |
TAT is an extremely valuable precursor of HMX which can be easily prepared by acetylation of DAPT in presence of acetic anhydride and anhydrous sodium acetate at 110oC in high yield.
Nitrolysis of TAT to HMX involving the reaction of TAT with a mixture of nitric acid and phosphorus pentoxide at 75oC for 15 minutes crude HMX is obtained in 75-80% yield with impurities such as SOLEX (1-acetyl-3,5,7-trinitro-1,3,5,7-tetrazacyclooctane) which is the main by-product of this process . In order to improve the yield and purity of the product in the present work we changed the condition of the synthesis and replaced the nitric acid with RFNA, resulted pure product and more than 95% yield. HMX with high purity plays an important role in oil well drilling process and some other important non-military industrial uses.
Experimental
All starting chemicals were purchased from commercial suppliers and without further purification. The melting points are reported without correction.
Instruments
Melting points were determined on an Electrothermal9100 apparatus and were uncorrected. FTIR spectra were obtained on a Perkin-Elmer infrared spectrometer. 1H-NMR and 13C-NMR spectra were run on Bruker DRX-300 AVANCE spectrometers at 300 MHz for 1H-NMR, 75 MHz for 13C-NMR. DMSO-d6 was used as solvent. The purification of HMX was done using preparative HPLC (column C18, 5 μm and mobile phase, MeOH: H2O).
General procedure for the Nitrolysis of TAT to HMX
The procedure started by stirring RFNA (30ml) in 5oC and P2O5 (12gr.) added gradually to the reaction media while the temperature was fixed at 5oC, after adding TAT to the solution temperature increased to 75oC and the mixture stirred for 15 min at 75 oC. after that the reaction solution was quenched in crushed ice (200 gr.) and pure HMX precipitated and filtered, washed with water and dried to 0.99 gr of HMX, yield 95%, m.p. 275oC (lit.[10] mp 274-276oC), 1H NMR (DMSO-d6,300 MHz) δ 6.2 (s, all protons); 13C NMR (DMSO-d6, 75 MHz) δ 64 ; the purity was 100% identified on HPLC.
Results and Discussion
In this paper we used a novel system for nitrolysis of TAT to HMX with the highest yield compared to other works reported in literatures and the highest purity.
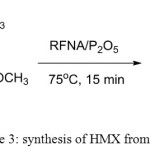 |
Figure 3: Synthesis of HMX from TAT Click here to View figure |
The reaction (figure 3) condition where optimized by changing time, temperature and mole ratio of RFNA/P2O5, the results are summarized in Table 1.
Table 1: Finding the optimized condition for HMX nitrolysis
|
Entry |
RFNA |
P2O5 |
Temperature(oC) |
Time(Min.) |
Yield |
m.p. |
|
1 |
30 |
0 |
75 |
15 |
X |
– |
|
2 |
30 |
10 |
75 |
15 |
87 |
265 |
|
3 |
30 |
14 |
75 |
15 |
88 |
273 |
|
4 |
30 |
8 |
75 |
15 |
66 |
267 |
|
5 |
20 |
8 |
75 |
15 |
55 |
274 |
|
6 |
25 |
10 |
75 |
15 |
62 |
275 |
|
7 |
30 |
12 |
85 |
15 |
65 |
275 |
|
8 |
30 |
12 |
60 |
15 |
60 |
262 |
|
9 |
30 |
12 |
75 |
10 |
65 |
272 |
|
10 |
25 |
10 |
75 |
25 |
70 |
275 |
|
11 |
30 |
12 |
75 |
25 |
90 |
267 |
|
12 |
35 |
14 |
75 |
15 |
67 |
265 |
|
13 |
30 |
12 |
75 |
15 |
95 |
275 |
According to the results, Low yields of HMX were obtain with lower amount of RFNA or P2O5 and lower ratio of RFNA/P2O5 made an obvious decline in the result of the reaction respectively 62% and 55%, after finding the optimal molar ratio influence of time and temperature were examined which showed that reducing the time of the reaction to less than 15min dropped the yield and increasing the time didn’t improve the yield of production.
In order to explain the mechanism of this reaction and the high yield and purity, we can consider the role of two sources of nitration agents (N2O5, NO2+), first source of nitration agents is P2O5 and nitric acid [11] which produce phosphoric acid as a by-product. The second source of producing nitration agents, is N2O4 (in RFNA) [12] at presence of the phosphoric acid which is the by-product of the first reaction. This concentrated amounts of nitration agents could turn all of the acetyl groups on TAT to nitrogen dioxide at 75oC and completes the reaction to the highest yield and purity.
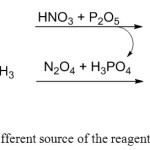 |
Figure 4: Two different source of the reagents for TAT nitrolysis Click here to View figure |
Conclusion
In conclusion, the RFNA/P2O5 mixture is a powerful and efficient reagent in the ambient time and temperature for nitrolysis of TAT to the valuable HMX, therefore this procedure has the highest yield reported in literatures which is more than 95% of HMX per mole of TAT and pure HMX which means no by-products such as RDX and SOLEX. short reaction time, excellent yield and purity, easy work-up are among the advantages of this procedure.
References
- Hua, Q.; Zhiewn, Y.; Chunxu, L. Ultrason. Sonochem. 2008, 15(4), 326.
CrossRef - Bayat, Y.; Mostafavi, M. M. B. Kor. Chem. Soc. 2012, 33, 3552.
- Yi, W. B.; Cai, C. Prop. Exp. Pyro. 2009, 34, 161.
CrossRef - Zhi, H. Z.; Luo, J.; Feng, G. A.; Lv, C. X. Chinese. Chem. Lett. 2009, 20, 379.
CrossRef - Lukasavage, W. J.; Behrmann, L. A.; Voreck, W. E. WO Patent 20000733285, 2000.
- Greenfield, M.; Guo, Y. Q.; Bernstein, E. R. Chem. Phys. Lett. 2006, 430, 277.
CrossRef - Siele, V. I.; Gilbert, E. E. US Patent 3939148, 1976.
- Bachmann, W. E.; Sheehan, J. C. J. Am. Chem. Soc. 1949, 71, 1842.
CrossRef - Siele, V. I.; Warman, M.; Leccacorvi, J.; Hutchinson, R. W.; Motto, R.; Gilbert, E. Prop.Explos. Pyro. 1981, 6, 67.
CrossRef - Ampleman, G.; Marois, A.; Thiboutot, S.; Hawari, J.; Greer, C.W.; Godbout, J.; Sunahara, G. I.; Shen, C. F.; Guiot, S. R. J. Labelled Compd. Radiopharm. 1999, 42, 1251.
CrossRef - Millar, R. W.; Colclough, M. E.; Golding, P.; Honey, P. J.; Paul, N. C.; Sanderson, A. J. Philos. T. Roy. Soc. A, 1992, 339.
- Olah, G. A.; Malhotra, R.; Narang, S. C. “Nitration: methods and mechanisms” (1989).

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









