The Effects of Pretreatment Methods of Carbon-Containing Wastes in Thermal Catalytic Recycling
Zheneta Tashmukhambetova1, Yermek Aubakirov1, Zhanat Shomanova2, Kairat Burkhanbekov1, Ruslan Safarov3, Larissa Sassykova1 , Nurbubi Zhakirova1 and Maria Faizullaeva4
, Nurbubi Zhakirova1 and Maria Faizullaeva4
1Al-Farabi Kazakh National University, 71, al-Farabi ave., 050040, Almaty, Kazakhstan.
2Pavlodar State Pedagogical Institute, Pavlodar, Kazakhstan.
3L.N.Gumilyev Eurasian National University, Astana, Kazakhstan.
4Scientific Research Institute for New Chemical Technologies and Materials, 95a, Karasaibatyr str., 050012, Almaty, Kazakhstan.
Corresponding author Email: larissa.rav@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/330622
This paper represents the results of studies of the influence of non-traditional pretreatment methods of carbon-containing wastes (used tires and waste plastics) by sonication, radiation exposure, treatment with liquid nitrogen and grinding for thermal catalytic recycling in the presence of heavy oil residue. The processes were carried out with composite catalysts based on ferroalloy production waste and natural zeolite in 40:60 and 60:40 wt. % proportions. The sonication of tire and plastic crumbs were done in the range of intensity I = 1-5 W /cm2 and t = 50-150 sec time and didn’t give clear patterns about the yields of gaseous and liquid products. Pretreatment of the waste samples with irradiation was examined between 100 to 500 kGy doses and their thermal catalytic recycling had shown increasing of gases about 4-5 wt. % and decreasing of liquid fractions approximately 8-10 wt. %. By the results of pretreatment with liquid nitrogen was found that mainly external changes occur on waste tire and due to this for the grinding method was chosen just the tire sample. The thermal catalytic recycling of ground tire sample and waste plastic had illustrated the highest yield of liquid products compared to other methods of pretreatment.
KEYWORDS:pre-treating; sonication; irradiation; liquid nitrogen; catalyst
Download this article as:| Copy the following to cite this article: Tashmukhambetova Z, Aubakirov Y, Shomanova Z, Burkhanbekov K, Safarov R, Sassykova L, Zhakirova N, Faizullaeva M. The Effects of Pretreatment Methods of Carbon-Containing Wastes in Thermal Catalytic Recycling. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Tashmukhambetova Z, Aubakirov Y, Shomanova Z, Burkhanbekov K, Safarov R, Sassykova L, Zhakirova N, Faizullaeva M. The Effects of Pretreatment Methods of Carbon-Containing Wastes in Thermal Catalytic Recycling. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40961 |
Introduction
In our modern world, the production of industrial and household materials extremely increases. It has resulted in a large quantity of modern wastes as used tires, plastics and other polymeric materials1. The main problem, these wastes are hard biodegradable2,3. So, waste disposal problems of carbon-containing materials and the aims of their large-scale secondary usage require appropriate technological solutions. This problematic situation is an actual trend for the scientific development for the production.
Pre-treatment of carbon-containing wastes with known physical and chemical methods can have a significant impact on changing their structure and hydrocarbon composition and in some cases, even on reactivity, which enables to improve technologies for recycling of these wastes and increase the selectivity to the desired products.
In this work were investigated nontraditional pre-treatment methods for waste tires and plastics by sonication, radiation exposure, treatment with liquid nitrogen and grinding in order to increase the yield of liquid products and improving their qualitative compositions in thermal catalytic recycling with heavy oil residue. By the reason that heavy oil residue, oily sludge etc are largest categories of wastes generated by oil industry petroleum production and petroleum refinery plant and they are wastes that do not effectively treated4,5. Also, a heavy oil residue or paste agent (PA) can provide a good solvency for feedstock and be a hydrogen donor for the product.
Instead of catalyst of the process was used a composite based on ferroalloy production waste (FPW)6 and natural zeolite.
Materials and Methods
Experiments for the waste plastic recycling and on its mixture with used tire in proportion of 1:1 were carried out on a batch type installation under initial 0.5 MPa pressure of argon and at a temperature of 450 °C with continuous mixing mode for 15 minutes7,8. For experiments only with waste tire crumbs, the temperature and time were 400°C and 60 minutes respectively. A ratio of the PA and feedstock was 1:1 and the weight of catalyst was 2% of the total amount of feedstock. Then, the obtained synthetic oil after each experiment was subjected to distillation under atmospheric pressure and fractions boiling at temperatures before 180, from 180 to 250 and from 250 to 320 °C were isolated. The formed gas was collected in a gas holder9.
The source of hydrogen and the binder of the feedstocks was a paste based on heavy oil residues from the “Kumkol” oil field (Southern Kazakhstan)10, with boiling points above 500 °C.
Composite catalysts were prepared by mixing the waste product from a ferroalloy plant (Aksu ferroalloy plant, Pavlodar region, Kazakhstan)11 and thermally activated natural zeolite from the “Tayzhuzgen” field (Eastern Kazakhstan) in 40:60 and 60:40 wt. % proportions11.
The sonocation was performed in a multifunctional ultrasonic laboratory complex MARK-3/22-AL (U-Sonic Russia).
Radiation treatment of tire crumbs was carried out on a linear electron accelerator ELA-6 with energy 6-MeV in an inert environment. The irradiation was performed with a flux density of electrons 2 uA/cm2.
Waste treatment with liquid nitrogen was performed in the Dewar vessel for several days.
The grinding was done in a planetary ball mill Pulverisette 5/4 classic line.
The received liquid products were investigated by means of the 6890N/5973N gas-liquid chromatograph with a mass-spectrometer detector.
Results and Discussion
As known, the main parameters for the selection of catalysts are their catalytic activity, selectivity and stability. All heterogeneous catalysts utilized in thermal processes have problems with rapid deactivation. When, synthetic aluminosilicate zeolite used as a catalyst for the thermal processing of wastes encounters difficulty with separation from the solid carbonaceous residue of the process. Therefore, using cheap and affordable natural aluminosilicates12 as catalysts, which have a high activity and selectivity, will significantly reduce the economic costs of these processes. For this reason, our catalysts for the thermal processing of used tires and plastics were composed from FPW and natural zeolite.
The morphology of samples of catalysts by method of scanning electron microscopy using the JEOL JSM 6460LV electron microscope with a tungsten thermionic cathode with an electron beam energy of 20 keV had been studied (fig.1,2).
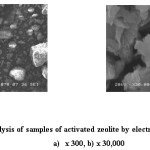 |
Figure 1: Analysis of samples of activated zeolite by electron microscopy: х 300, b) х 30,000 Click here to View figure |
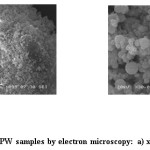 |
Figure 2: Analysis of FPW samples by electron microscopy: a) x 3,000, b) x 30,000 Click here to View figure |
As can be seen from fig.1, in a zeolite activated by acid treatment at a 300-fold magnification (a), structures that are fairly heterogeneous in degree of dispersion are seen, which, as follows from picture (b), at 30,000-fold magnification, represent a fairly uniformly layered surface.
It follows from fig.2, the FPW catalyst structure at 3,000x magnification (a) is represented mainly by amorphous rounded particles, which are clearly visible at a 30,000-fold increase (b).
The structure of the catalyst is rather loose with a large specific surface area. Both samples proposed by us as catalysts have a sufficiently developed specific surface, which should promote a more active and possibly selective transformation of the organic mass of waste tires and plastics into liquid products.
Thus, the complex of analyzes of composition and physicochemical properties allows us to conclude that the samples on the basis of polymetallic wastes of ferroalloys and activated natural zeolite are of interest in their elemental composition and surface structure as available, relatively cheap and active catalysts for the process Hydrogenation thermocatalytic processing of carbonaceous wastes based on worn tires and plastics in motor fuels. At the same time, the issues of rational use of not only carbon-containing raw materials, but also industrial polymetallic wastes will be resolved.
Before thermal processing of our selected wastes they were subjected to the pre-treating methods as sonication, radiation exposure, treatment with liquid nitrogen and grinding.
Sonication effect
As known, the ultrasound can’t do a direct influence to the chemical change of molecules, because its wavelength is too long compared to the molecules13. So that, the energy goes to creat a cavitation effect, which generates extremes of temperature and pressure in the liquid where the reaction happens. Also, ultrasound breaks up a dispersible substance into tiny microparticles and gives them a larger surface area for further chemical reactions.
The sonication of tire and plastic crumbs with particle sizes of 0.9, 1.9, 2.9 and 3.9 mm were done in water. After, they were tested in thermal processing with FPW-zeolite catalyst in the ratio of 40:60.
Variation of the ultrasonic treatment parameters in the range of intensity values (I) of 1-5 W/cm2 and time (τ) of 50-150 sec (tab.1) doesn’t have a significant impact on the dispersion and structural changes of tire and plastic crumbs. Previously, we have established that tire crumbs with 0.6-0.8 mm particle sizes in ultrasonic treatment in an aqueous medium have no significant effect on the yield of liquid products and their fractional compositions.
Table 1: The yield of liquid products in the thermal catalytic recycling of ultrasonic treated mixtures of tire and plastic crumbs
|
I, W/cm2 |
t, sec |
Yield of gas (wt. %) |
The yield of liquid products, wt. % |
Yield of solid residue, (wt. %) |
Losses, (wt. %) |
|||
|
untill 180 оС |
180-250 оС |
250-320 оС |
∑LP |
|||||
|
1 |
50 |
17.60 |
18.60 |
18.70 |
19.80 |
57.10 |
17.43 |
7.87 |
|
100 |
16.63 |
20.00 |
23.10 |
14.90 |
58.00 |
11.45 |
13.92 |
|
|
150 |
16.30 |
18.40 |
19.90 |
21.60 |
59.90 |
12.89 |
10.91 |
|
|
3 |
50 |
11.70 |
15.20 |
25.10 |
14.10 |
54.40 |
19.36 |
14.54 |
|
100 |
14.20 |
18.70 |
14.80 |
22.90 |
56.40 |
17.53 |
11.87 |
|
|
150 |
13.42 |
20.80 |
17.30 |
19.80 |
57.90 |
16.47 |
12.21 |
|
|
5 |
50 |
14.92 |
24.40 |
22.60 |
10.12 |
57.12 |
19.50 |
8.46 |
|
100 |
17.40 |
18.60 |
26.90 |
13.50 |
59.00 |
11.72 |
11.88 |
|
|
150 |
15.70 |
23.00 |
27.60 |
10.40 |
61.00 |
13.81 |
9.49 |
|
Also, a separate ultrasonic treatment of plastic crumbs in water with particle sizes of 2.0, 4.0 and 6.0 mm was impractical, because, the sonication doesn’t have impact on so strong, greasy and adhesively resistant material14. In order to find the influence of the ultrasonic treatment on the yield and composition of the liquid products, experiments were done to the mixtures of used automobile tire and plastic crumbs in ratios of 1:1 and with particle sizes 2.0-6.0 mm.
As can be seen from the data of tab.1, testing the mixtures of used tire and waste plastics at I = 1, 3, 5 W/cm2 and with processing time of 50, 100, 150 seconds in room temperature doesn’t give clear patterns about the yields of gaseous and liquid products.
The total yields of liquid products were in the range of 54.4-61.0 wt. %. Likewise, yields of gases were between 11.7-17.6 wt. % and as can be seen from Table 1 it slightly depends on the ultrasound intensity and processing time.
Mainly, sonication influences to the waste tire crumbs structure, which is accompanied by a mechanical rupture of associatively linked molecules of the tire and vulcanized sulfur. Moreover, it impacts to the separation of metal cord, carbon black and others organic fillers from tire composition, which should increase the converting of the rubber into liquid and gaseous products. It was found that the ultrasound treatment with I = 5 W/cm2 intensity and t = 150 sec timegives the highest yield of liquid products, which is 61.0 wt. %.
Radiation exposure effect
Also, in this work were studied the effect of radiation exposure of used tires and waste plastics to the yield and composition of the liquid products obtained by their thermal catalytic recycling.
The dose of irradiation ranged from 100 to 500 kGy and the FPW-zeolite catalyst in the ratio of 40:60 was used in the process.
Table 2: The yield of liquid products in the thermal catalytic recycling of irradiated mixtures of tire and plastic crumbs
|
Irradiation dose, kGy |
Yield of gas (wt. %) |
The yield of liquid products, wt. % |
Yield of solid residue, (wt. %) |
Losses, (wt. %) |
|||||||
|
untill 180 оС |
180-250 оС |
250-320 оС |
∑LP |
||||||||
|
100 |
21.41 |
11.68 |
19.72 |
17.11 |
48.51 |
25.71 |
4.37 |
||||
|
200 |
16.43 |
11.98 |
14.43 |
11.74 |
38.15 |
26.92 |
18.50 |
||||
|
300 |
10.56 |
10.20 |
12.72 |
20.47 |
53.39 |
27.77 |
8.28 |
||||
|
400 |
18.95 |
6.23 |
8.72 |
17.23 |
32.18 |
25.19 |
23.68 |
||||
|
500 |
21.80 |
15.11 |
3.14 |
15.15 |
33.40 |
22.39 |
22.41 |
||||
As seen from the tab.2, the total yield of liquid products from the irradiated mixture of used tires and waste plastics changes between 32.18-53.39 wt. %. In the ranges of 100 and 300 kGy irradiations are observed high yields of the liquid fractions (fig.3). The optimal yield for the first fraction before 180 °C is observed at the irradiation dose of 500 kGy and for the third fraction between 250-320 °C at 300 kGy. Also, the total highest yield of the liquid products was shown with 300 kGy irradiation dose.
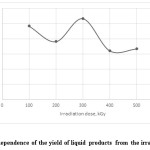 |
Figure 3: The dependence of the yield of liquid products from the irradiation dose Click here to View figure |
It should be noted that the irradiation of worn tires and waste plastics in the investigated intervals does not provide significant effect on the yield of light distillates in a thermal catalytic recycling. The yield of gas has slightly increased about 4-5 wt. % and liquid fractions decreased about 8-10 wt. %. This is consistent with the published data that the preliminary radiation of carbon-containing materials as rubber and plastic significantly increases the yield of gases. During irradiation occurs a destructive splitting of bonds in the rubber and polymer cord, which leads to further easier thermal splitting of molecules under the influence of high temperature to the gases of C1-C4, carbon oxides and also to the formation of sulfur-containing gases. Mainly, the heavy oil residue makes a significant contribution to the formation of the synthetic oil.
A gas-chromatographic analysis of the liquid products has shown the presence of C1-C4 normal and isoparaffinic hydrocarbons, olefins and dienes. The gas composition was illustrated with C4-C5, H2, CO, CO2 and H2S compounds.
Treatment with liquid nitrogen
By using a cryogenic method for the pretreatment of used tires and waste plastics was found, that mainly external changes were on the tire sample, which is probably due to structural changes in its organic part. Also, pre-treating of the tire sample with liquid nitrogen has resulted to the mechanical separation of rubber and polymer fibers from the metal cord and other constituents. After drying in a vacuum oven, the rubber sample was separated again from the visible fibers and subjected to the mechanical grinding in a ball mill.
Grinding effect
Due to the results of treating with liquid nitrogen, for the grinding method was chosen just the tire sample. Then, in ball mill the tire sample was ground into particle sizes of 0.5-0.7, 0.8-1.0, 2.0-4.0 and 5.0-7.0 mm. After, the dispersed tire samples were tested in thermal catalytic recycling with FPW-zeolite catalyst in the ratio of 60:40.
Table 3: The dependence of the yield of liquid products from the dispersion degree
|
Dispersion degree, mm |
Yield of gas (wt. %) |
The yield of liquid products, wt. % |
Yield of solid residue, (wt. %) |
Losses, (wt. %) |
|||
|
untill 180 оС |
180-250 оС |
250-320 оС |
∑LP |
||||
|
0.5-0.7 |
11.43 |
12.59 |
17.89 |
25.45 |
55.03 |
27.51 |
6.03 |
|
0.8-1.0 |
11.88 |
13.45 |
17.49 |
24.19 |
55.13 |
25.43 |
7.56 |
|
2.0-4.0 |
9.28 |
14.09 |
19.13 |
22.01 |
55.23 |
28.07 |
7.42 |
|
5.0-7.0 |
9.51 |
13.76 |
19.07 |
23.23 |
56.06 |
27.65 |
6.78 |
It is shown that the catalytic activity of the used catalyst rises with increasing particle size of rubber crumbs, which is apparently due to the formation of optimal contact surfaces between catalyst particles and feedstock in the given process conditions (Table 3). The yield of liquid products varies approximately on 3 wt. % and it is in the range of 54.13-56.06 wt. %.
Thus, pre-treating of the used tire with liquid nitrogen and its further mechanical grinding into different particle sizes doesn’t substantially affect to the fractional composition and yields of the liquid products, although, it promotes to the easier separation of rubber from the inorganic compounds and soot. Earlier we have shown that tire crumbs with particle sizes of 0.4-0.6 mm gives about 56.8 wt. % yields of liquid products15,16.
In the composition of the waste tire sample with 0.5-0.7 mm particle sizes were found about 62 wt. % of rubber (natural and butadiene-styrene), 3 wt. % of a carbon black and 35 wt. % of other constituents.
The waste polymer sample after 1-3 days treatment with liquid nitrogen almost hadn’t any changes. Then, it was subjected to the thermal catalytic recycling with already ground tire sample (5.0-7.0 mm) and without it (tab.4).
Table 4: The yield of liquid products from the thermal catalytic recycling of waste plastic with waste tire and without it
|
Process |
Yield of gas (wt. %) |
The yield of liquid products, wt. % |
Yield of solid residue, (wt. %) |
Losses, (wt. %) |
|||
|
untill 180 оС |
180-250 оС |
250-320 оС |
∑LP |
||||
|
With plastic |
2.91 |
10.89 |
18.43 |
37.69 |
67.01 |
12.04 |
18.04 |
|
Plastic-tire |
4.91 |
24.91 |
25.08 |
15.98 |
65.97 |
13.09 |
16.03 |
As seen from the tab.4, total yields of liquid products in both processes almost the same, about 67.01 wt. % with plastic and 65.97 wt.% with the mixture of plastic and tire crumbs. But, these results are higher than other experiments. It means treatment with liquid nitrogen of carbon-containing wastes and their further grinding in ball mills into known particle sizes is an optimal by compare with other non-traditional methods of pretreatment.
So, in this work was shown clear patterns about the effects of non-traditional methods of pretreatment of carbon-containing wastes in the thermal catalytic recycling. By these pre-treating methods it is possible to get more yields of liquid products and get some structural changes in their composition. According to the results, we can say that not all the methods of pretreatment of carbon-containing wastes are effective for the yield of liquid products in thermal catalytic processing.
Conclusion
It is established, mainly, sonication influences to the waste tire crumbs structure, which is accompanied by a mechanical rupture of associatively linked molecules of the tire and vulcanized sulfur.
Pretreatment of used tire and waste plastic with irradiation had led to the increasing of gases about 4-5 wt. % and decreasing of liquid fractions approximately 8-10 wt. %. It is due to destructive splitting of bonds in the rubber and polymer cord and their converting into carbon oxides, C1-C4 hydrocarbons and also to the sulfur-containing gases by temperature.
By the results of pretreatment with liquid nitrogen was found that mainly external changes occur on waste tire, due to this, the tire was subjected to the mechanical grinding.
The ground tire sample had illustrated the highest yield of liquid products in thermal catalytic recycling compared to other methods of pretreatment. The mixture of ground tire and waste plastic had shown the highest yield of liquid products compared to other methods of pretreatment.
According to the results, we can say that not all the methods of pretreatment of carbon-containing wastes are effective for the yield of liquid products in thermal catalytic processing. Also, it is clear, that these pretreatment methods are more effective for used tires than for waste plastics.
References
- Oyedun, A.; Lam, K.; Fittkau, M.; Hui, Ch. Fuel 2012, 95, 417-424.
CrossRef - Uçar, S.; Karagöz, S.; Yanik, J.; Saglam, M.; Yuksel, M. Fuel Processing Technology 2005, 87, 53-58.
CrossRef - Miranda, M.; Pinto, F.; Gulyurtlu, I.; Cabrita, I.; Fuel, 2013, 103, 542-552.
CrossRef - Sendilvelan, S.; Bhaskar, K. Rasayan J.Chem. 2016, 9(4), 692-696.
- Jinsheng, Zh.; Minghua, Zh. Oxidation Communications, 2015, 38 (4), 1813.
- Shomanova, Zh.; Safarov, R.; Nosenko, Y.; Tashmukhambetova, Zh. Conference Proceedings, Barcelona Spain, 2016, 18(10), Part 1, 71-75.
- Tashmukhambetova, Zh.Kh.; Kairbekov, Zh.K.; Aubakirov, E.A.; Burkhanbekov, K.E.; Faizullaeva, M.F.; Shomanova, Zh.K. Solid Fuel Chemistry, 2016, 50(4), 220-225.
CrossRef - Aubakirov, E.; Tashmukhambetova, Zh.; Kairbekov, Zh.; Burkhanbekov K. Applied Mechanics and Materials, 2014, 618, 136-139.
- Aubakirov, E. A.; Tashmukhambetova, Zh. Kh.; Burkhanbekov, K. E. Scientific Journal of the Modern Education and Research Institute, The Kingdom of Belgium, Special Edition, 2016, 88-94.
- Shomanova, Zh. K.; Tashmukhambetova, Zh. Kh.; Safarov, R. Z.; Nosenko, Yu. G.; Kaliakparov, A. G.; Shomanov, A. S. News of the Academy of Science of the Republic of Kazakhstan, 2013, 397(1), 35-41.
- Kairbekov, Zh. K.; Aubakirov, E. A.; Tashmukhambetova, Zh. Kh.; Fayzullaeva, M. F.; Shomanova, Zh. K.; Burkhanbekov, K. E. Bulletin of the KazNU, Chemical Series, 2015, 77a(1), 90-95.
- Suvorov, Yu. P., Solid Fuel Chemistry, 2006, 6, 57–62.
- Sendilvelan, S; Bhaskar, K. Orient. J. Chem. 2017, 33(4), 2111-2117.
CrossRef - Bergman, L. Ultrasound and Its Application in Science and Technology, Moscow, 1956
- assykova, L.; Nalibayeva, A; Aubakirov, Y.; Tashmukhambetova, Zh.; Otzhan, U; Zhakirova, N.; Faizullaeva, M., Orient. J. Chem. 2017, 33(4), 1941-1948.
- Kairbekov, Zh. K.; Aubakirov, E. A.; Tashmukhambetova, Zh. Kh.; Burkhanbekov, K. E.; Abildin, T.S., Bulletin of the KazNU, Chemical Series, 2015, 77а (1), 96-101.

This work is licensed under a Creative Commons Attribution 4.0 International License.









