Synthesis and Free Radical-Scavenging Activities of Di-Mannich Bases of Cyclovalone Derivatives
Hayun Hayun , Catur Jatmika, Euis Maras Purwati, Sandi Salim, Rosita Kurniawan, Elizabeth Greffiana Chandra, Adam Arditya Fajriawan and Aulika Desthahrina Nareswara
, Catur Jatmika, Euis Maras Purwati, Sandi Salim, Rosita Kurniawan, Elizabeth Greffiana Chandra, Adam Arditya Fajriawan and Aulika Desthahrina Nareswara
Faculty of Pharmacy, Universitas Indonesia, Depok, West Java,16424, Indonesia.
Corresponding Author E-mail: hayun.ms@ui.ac.idDOI : http://dx.doi.org/10.13005/ojc/330607
Novel di-Mannich bases of cyclovalone derivatives (1) have been synthesized and evaluated their antioxidant activity using DPPH free radical-scavenger method. The structures of the compounds were confirmed on the basis of FT-IR, 1H-NMR, 13C-NMR and mass spectral data. The result of antioxidant evaluation showed that di-Mannich derivative of cyclovalone with diethylamine ((2E,6E)-2,6-bis({3-[(diethylamino)methyl]-4-hydroxy-5-methoxyphenyl}methylidene)cyclohexan-1-one) (2a) exhibited the highest antioxidant activity with IC50 = 39.0 µM. Structure-activity relationship study showed that the higher pKa of the Mannich base, the higher activity (the lower IC50) of the compound.
KEYWORDS:Cyclovalone; Mannich base; Synthesis; Antioxidant; Radical scavenger
Download this article as:| Copy the following to cite this article: Hayun H, Jatmika C, Purwati E. M, Salim S, Kurniawan R, Chandra E. G, Fajriawan A. A, Nareswara A. D. Synthesis and Free Radical-Scavenging Activities of Di-Mannich Bases of Cyclovalone Derivatives. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Hayun H, Jatmika C, Purwati E. M, Salim S, Kurniawan R, Chandra E. G, Fajriawan A. A, Nareswara A. D. Synthesis and Free Radical-Scavenging Activities of Di-Mannich Bases of Cyclovalone Derivatives. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40849 |
Introduction
Oxidative stress, generating by an imbalance between free radical production and antioxidant defenses, is associated with the damage of various species of molecules including proteins, lipids, and nucleic acids. A role for oxidative stress has been postulated to contribute significantly to the pathogenesis of atherosclerosis, inflammatory conditions, certain cancers, and the process of aging. Besides the complex system of antioxidant metabolites and enzymes that naturally prevent cell damage, the exogenous antioxidant may sometimes be required to keep reactive oxygen species at optimum level 1-3.
Cyclovalone, (2E,6E)-2,6-bis[(4-hydroxy-3-methoxyphenyl)methylidene]cyclohexan-1-one (1) is a curcumin mono-carbonyl analog in which the pentane-2,4-dione chain of the curcumin is replaced by a cyclohexanone ring. Cyclovalone demonstrated antioxidant activity, antitumor, anti‐inflammatory, hepatoprotective and cytotoxic activity4-7. The structure-activity relationships (SAR) including the antioxidant activity of cyclovalone derivatives have been studied. However, there has been no report about the effect of aminoalkyl substituents5,8.
A Mannich reaction is a suitable method to introduce aminoalkyl group into a molecule. In several instances, the Mannich derivatives exhibit better biological activity than the corresponding parent analogs. Moreover, the presence of Mannich side chain increases the solubility and hence the bioavailability of the compounds [2,9-13. Herein we report the synthesis and antioxidant activity of Mannich bases derivatives of cyclovalone.
Materials and Methods
Chemistry
All used chemicals were purchased from Merck or Aldrich Company and used without further purification. Thin layer chromatography (TLC) was carried out on silica gel 60 F254 plates (Merck) and spots were detected under an ultraviolet and visible light. Melting points were determined in the capillary tube using electrothermal digital melting point apparatus (Stuart Scientific). The infrared spectra were recorded with FTIR 8400S Spectrometer (Shimadzu). NMR spectra were recorded on NMR spectrometer (Agilent) at 500 MHz for 1H and 125 MHz for 13C using TMS as an internal standard. High-resolution mass spectra (HRMS) were measured with a Waters LCT Premier XE (ESI-TOF) system in positive or negative mode. The absorption in the determination of free radical-scavenging activity was measured using UV-Vis Spectrophotometer 1601 (Shimadzu).
General synthesis of di-Manncih bases of cyclovalone derivatives (2a-e).
The di-Mannich bases of cyclovalone were synthesized by Mannich reaction of compound 1 according to the method of synthesis of di-Mannich bases of 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one reported previously7, with little modification: The solution of 2 mmol of compound 1 in acetonitrile (50 ml) was added to mixture of 16 mmol paraformaldehyde and 16 mmol of corresponding secondary amine in acetonitrile (50 ml) which previously was heated at 80 oC for 10 min. The reaction mixture then was refluxed until the disappearance of 1. The completion of the reaction was monitored by TLC for 5-27 h. The reaction solvent was removed in vacuo using a rotary evaporator, the crude products were washed with cold acetonitrile and then purified by recrystallization or column chromatography to give compound 2a-e.
(2E,6E)-2,6-bis({3-[(diethylamino)methyl]-4-hydroxy-5-methoxyphenyl} methylidene)cyclohexan-1-one (2a):
The crude product was purified by recrystallization from ethylacetate-hexane (1:25) gave a yellowish orange crystalline powder at 84.3% yield, m.p. = 58-60 oC. IR (KBr), ῡmax, cm-1: 2972, 2935, 2833, 1639, 1589, 1246 1087, and 1039. 1H-NMR (500 MHz, CDCl3) δ/ppm: 7.71 (2H, s, C=CH-), 6.97 (2H, s, HAr), 6.80 (2H, s, HAr), 3.91 (6H, s, OCH3), 3.82 (4H, s, Ar-CH2-N-diethylamine), 2.94 (4H, t, =C-CH2-Ccyclohexanone), 2.66 (8H, q, -CH3–CH2-Ndiethylamine), 1.83 (2H, p, C-CH2-Ccyclohexanone) and 1.14 (12H, t, CH2–CH3 diethylamine). 13C-NMR (125 MHz, CDCl3) δ/ppm: 190 (1C, C=Ocyclohexanone), 149 and 147 (4C, CAr-O), 133 and 137 (4C, -C=C-), 126, 123, 121, and 113 (8C, CAr-H), 57 (2C, Ar-C-Ndiethylamine), 56 (2C, Ar-OCH3), 46 (4C, -N-CH2 diethylamine), 28 (2C, =C-CH2-Ccyclohexanone), 23 (1C, C-CH2-Ccyclohexanone), and 11 (C-CH3). HRESIMS (m/z): found 537.3315 ([M+H]+), calculated masses of C32H45N2O5: 537.3328 (error – 2.4 ppm).
(2E,6E)-2,6-bis({3-[(dimethylamino)methyl]-4-hydroxy-5-methoxyphenyl} methylidene)cyclohexan-1-one (2b):
The crude product was purified by column chromatography on silica with mixture of chloroform, methanol and ethanol (10:1:1) as mobile phase gave a brown crystalline powder at 44.3% yield, m.p. = 182-184 oC. IR (KBr), ῡmax, cm-1: 2993, 2829, 1605, 1558, 1488, 1250 1086, and 1008. 1H-NMR (500 MHz, CDCl3) δ/ppm: 7.70 (2H, s, C=CH-), 6.97 (2H, s, HAr), 6.79 (2H, s, HAr), 3.89 (6H, s, OCH3), 3.68 (4H, s, Ar-CH2-Ndimethylamine), 2.93 (4H, t, =C-CH2-Ccyclohexanone), 2.35 (12H, s, CH3-N-dimethylamine), and 1.82 (2H, p, C-CH2-Ccyclohexanone). 13C-NMR (125 MHz, CDCl3) δ/ppm: 190 (1C, C=Ocyclohexanone), 149 and 148 (4C, CAr-O), 134 and 137 (4C, -C=C-), 127, 124, 122, and 114 (8C, CAr), 63 (2C, Ar-C-Ndimethylamine), 56 (2C, Ar-OCH3), 45 (4C, -N-CH3 dimethylamine), 29 (2C, =C-CH2-Ccyclohexanone), and 23 (1C, C-CH2– Ccyclohexanone). HRESIMS (m/z): found 479.2539 ([M-H]–), calculated masses of C28H35N2O5: 479.2546 (error -1.5 ppm).
(2E,6E)-2,6-bis({[4-hydroxy-3-methoxy-5-(morpholin-4-ylmethyl)phenyl] methylidene})cyclohexan-1-one (2c):
The crude product was purified by recrystallization from ethylacetate-hexane (1:1) gave a yellow crystalline powder at 63% yield, mp = 172-174 °C. IR (KBr) ῡmax, cm-1: 2933, 2837, 1654, 1591, 1494, 1305, 1259, 1087, and 1001. 1H-NMR (500 MHz, CDCl3) δ/ppm: 7.68 (2H, s, C=CH-), 6.96 (2H, s, HAr), 6.80 (2H, s, HAr), 3.89 (6H, s, OCH3), 3.75 (12 H of 4H, Ar-CH2-Nmorpholine and 8H, C-CH2-Omorpholine), 2.91 (4H, t, =C-CH2-Ccyclohexanone), 2.59 (8H, t, C-CH2-Nmorpholine), and 1.81 (2H, p, C-CH2-Ccyclohexanone). 13C-NMR (125 MHz, CDCl3) δ/ppm: 190 (1C, C=Ocyclohexanone), 148 and 147 (4C, CAr-O), 137 and 134 (4C, -C=C-), 127, 124, 121, and 114 (8C, CAr), 67 (4C, C-O-C), 62 (2C, Ar-C-Nmorpholine), 56 (2C, Ar-OCH3), 53 (4C, C-N-Cmorpholine), 29 (2C, =C-CH2-Ccyclohexanone), and 23 (1C, C-CH2-Ccyclohexanone). HRESIMS (m/z): found 565.2916 ([M+H]+), calculated masses of C32H41N2O7: 565.2914 (error 0.4 ppm).
(2E,6E)-2,6-bis({3-[(2,6-dimethylmorpholin-4-yl)methyl]-4-hydroxy-5-methoxy phenyl}methylidene)cyclohexan-1-one (2d):
The crude product was purified by recrystallization from chloroform-methanol (1:3) gave a pale yellow crystalline powder at 79% yield, m.p. = 192-194 °C. IR (KBr), ῡmax, cm-1: 2972, 2939, 2821, 1589,1417, 1375, 1653, 1257, 1085 and 1145. 1H-NMR (500 MHz, CDCl3) δ/ppm: 7.69 (2H, s, C=CH-), 6.97 (2H, s, HAr), 6.79 (2H, s, HAr), 4,07 (2H, br, OH), 3.89 and 3.90 (two peaks of 6H, s, OCH3 of the two isomers), 3.71 (s, 4H, Ar-CH2-Nmorpholine), 3,52-3,82 (4H, m, C-CH(-O-)-Cmorpholine), 2.92 (4H, t, =C-CH2-Ccyclohexanone), 2.83, 2.63, 2.27 and 1.87 (four peaks of 8 H, C-CH2-N-morpholine of the two isomers), 1.81 (2H, p, C-CH2-Ccyclohexanone), 1.15 and 1.24 (two peaks of d, 12H, CH-CH3 of the two isomers). 13C-NMR (125 MHz, CDCl3) δ/ppm: 190 (1C, C=Ocyclohexanone), 148 and 147 (4C, CAr-O), 137 and 134 (4C, -C=C-), 127, 124, 121, 114 (8C, CAr), 72 (4C, C-O-C), 62 (2C, Ar-C-Nmorpholine), 59 (4C, C-N-Cmorpholine), 56 (2C, Ar-OCH3), 29 (2C, =C-CH2-Ccyclohexanone), 23 (1C, C-CH2-Ccyclohexanone), 19 (4C, CH3-Cmorpholine). HRESIMS (m/z): found 621.3546 ([M+H]+), calculated masses of C36H49N2O7: 621.3540 (error 1.0 ppm).
(2E,6E)-2,6-bis({4-hydroxy-3-methoxy-5-[(4-methylpiperazin-1-yl)methyl] phenyl}methylidene)cyclohexan-1-one (2e):
The crude product was purified by column chromatography on silica with mixture of chloroform and methanol (1.5:1) as mobile phase gave a brownish orange crystalline powder at 57.0% yield, m.p. = 163-165 °C. IR (KBr), ῡmax, cm-1: 2939, 2839, 2797, 1657, 1586, 1500, 1302, 1249, 1086 and 1006. 1H-NMR (500 MHz, CDCl3) δ/ppm: 7.69 (2H, s, C=CH-), 6.96 (2H, d, J=2 Hz, HAr), 6.80 (2H, d, J=2 Hz, HAr), 4.4 (2H, br, OH), 3.89 (6H, s, OCH3), 3.76 (4H, s, Ar-CH2-Npiperazine), 2.92 (4H, t, =C-CH2-Ccyclohexanone), 2.61 (16H, N-C-CH2-Npiperazine), 2.29 (6H, s, CH3-Npiperazine), 1.81 (2H, p, C-CH2-Ccyclohexanone). 13C-NMR (125 MHz, CDCl3) δ/ppm: 190 (1C, C=Ocyclohexanone), 148 and 147 (4C, CAr-O), 137 and 134 (4C, -C=C-); 127, 124; 121, and 114 (8C, CAr), 61 (2C, Ar-C-Npiperazine), 56 (2C, Ar-OCH3), 55 and 53 (8C, C-N-Cpiperazine), 46 (2C, CH3-Npiperazine), 29 (2C, =C-CH2-Ccyclohexanone); 23 (1C, C-CH2-Ccyclohexanone). HRESIMS (m/z): found 589.3411 ([M-H]–), calculated masses of C34H45N4O5: 589.3390 (error 3.6 ppm).
Free Radical-Scavenging Activity Evaluation
The antioxidant activities of Mannich bases of cyclovalone derivatives (2a-e) and cyclovalone (1) were evaluated by the free radical-scavenging activity of stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) according to the methodology described by Brand-Williams et al. with a little modification17-18. Quercetin was used as reference standard. The test or reference compounds were prepared at five different concentrations in methanol. The test or reference solution (0.5 mL) was mixed with 0.5 mL of DPPH radical solution 0.5 mM in methanol and then allowed to stand at room temperature for 30 min in a dark laboratory condition. The changes in color (from deep violet to light yellow) were measured at 517 nm. The mixture of of methanol (0.5 mL) and of sample solution (0.5 mL) serve as a blank. The control solution was prepared by mixing methanol (0.5 mL) and DPPH radical solution (0.5 mL). The experiment for each test compounds was performed in triplicate. The percent free radical-scavenging activity (% Scavenging) was calculated according to the following equation:
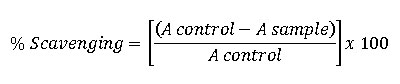
where A sample is the absorption of DPPH with test or reference compounds and A control is the absorption of DPPH without test compounds. Data obtained was then analyzed using a linear regression equation to determine IC50 of free radical-scavenging activity (FRSA) of the compounds.
Results and Discussion
Chemistry
The title compounds 2a-e were synthesized in two steps by the method summarized in Scheme 1. Vanillin was reacted with cyclohexanone according to the method previously reported to provide cyclovalone (1)5. Treatment of 1 with paraformaldehyde and corresponding secondary amine (a-e) in acetonitrile at reflux temperature for 5-27 h (TLC monitoring) afforded the title compounds 2a-e.
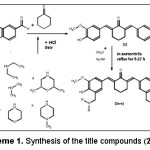 |
Scheme 1 Click here to View scheme |
The IR spectra of compounds 2a-e appeared CH aliphatic bands at 2,972-2,830 cm-1 and showed the disappearance of OH phenolic peak. The bands at 1,246-1,057 cm-1 and 1,000-1081 cm-1 correspond to C-O phenol, C-O ether, and C-N; while the α,β-carbonyl groups of the cyclovalone are observed as strong bands at 1,639–1,659 cm-1 and 1589-1591 cm-1. In 1H-NMR spectra, the protons of two symmetrical aromatic ring remained only four protons appeared at δ 6.9 ppm (2H) and at δ 6.8 ppm (2H) as singlet or doublet with J=1-2 Hz indicated that the Mannich base substituted a proton at the ortho position relative to the hydroxyl group of 1. The data were supported by the disappearance of OH phenolic peak in IR spectra caused by intramolecular hydrogen bond formation between the hydroxyl group and N atom of the Mannich base14-15. The structures were further supported by 13C-NMR and MS spectra of the compounds which showed the complete agreement with the assigned molecular structures.
Antioxidant acitivity
The antioxidant activities of the synthesized compounds were evaluated using DPPH free radical-scavenger method because of the suitability of the antioxidant mechanism with the compounds. Furthermore, the DPPH analysis is a fast and an uncomplicated test ensuring the reliable result. The mechanism of antioxidant activity briefly is that the phenolic compounds act as the hydrogen donor to reduce the radical molecules, and then the radical antioxidant will be stabilized by electron delocalization in the aromatic system or coupled with other or the same radical antioxidant to give non-radical molecules3,8,17-19.
The results of DPPH free radical-scavenging activity of the title compounds are listed in Table 1. All the compounds show free radical-scavenging activity. Compound 2a is the most potent, with IC50 = 39.0 µM or about a half of quercetin’s antioxidant activity. The activity of compound 2a and 2b are more potent than that of 1, while compound 2e, 2d and 2c are lower than that of 1. Structure-activity relationship study of the compounds demonstrates the effect of the alkalinity (pKa) of Mannich substituent of the compound on the antioxidant activity (Figure 1). The higher pKa of the Mannich base compound, the higher activity (the lower IC50) of the compound. In the previous study was reported that the hydroxyl group of the phenolic antioxidant compound is essential for the activity. The small alkyl substituents (e.g. methyl and ethyl) and electron donating groups (e.g. methoxy) at ortho positions relative to the phenol groups enhanced the activity. On the contrary, bulky alkyl substituents (e.g. isopropyl and t-butyl) retarded the activity5,8.
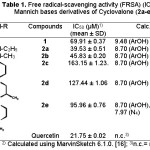 |
Table 1: Free radical-scavenging activity (FRSA) (IC50) of Mannich bases derivatives of Cyclovalone (2a-e) |
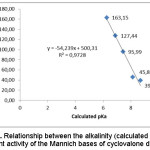 |
Figure 1: Relationship between the alkalinity (calculated pKa) and antioxidant activity of the Mannich bases of cyclovalone derivatives Click here to View figure |
![(2E,6E)-2,6-bis({3-[(diethylamino)methyl]-4-hydroxy-5- methoxyphenyl} methylidene)cyclohexan-1-one (2a):](http://www.orientjchem.org/wp-content/uploads/2017/12/Vol32_No6_Syn_HAY_Fig2-150x150.jpg) |
Figure 2: (2E,6E)-2,6-bis({3-[(diethylamino)methyl]-4-hydroxy-5- methoxyphenyl} methylidene)cyclohexan-1-one (2a): Click here to View figure |
![(2E,6E)-2,6-bis({3-[(dimethylamino)methyl]-4-hydroxy-5-methoxyphenyl} methylidene)cyclohexan-1-one (2b):](http://www.orientjchem.org/wp-content/uploads/2017/12/Vol32_No6_Syn_HAY_Fig3-150x150.jpg) |
Figure 3: (2E,6E)-2,6-bis({3-[(dimethylamino)methyl]-4-hydroxy-5-methoxyphenyl} methylidene)cyclohexan-1-one (2b): Click here to View figure |
![(2E,6E)-2,6-bis({[4-hydroxy-3-methoxy-5-(morpholin-4-ylmethyl)phenyl] methylidene})cyclohexan-1-one (2c):](http://www.orientjchem.org/wp-content/uploads/2017/12/Vol32_No6_Syn_HAY_Fig4-150x150.jpg) |
Figure 4: (2E,6E)-2,6-bis({[4-hydroxy-3-methoxy-5-(morpholin-4-ylmethyl)phenyl] methylidene})cyclohexan-1-one (2c): Click here to View figure |
![(2E,6E)-2,6-bis({3-[(2,6-dimethylmorpholin-4-yl)methyl]-4-hydroxy-5-methoxy phenyl}methylidene)cyclohexan-1-one (2d):](http://www.orientjchem.org/wp-content/uploads/2017/12/Vol32_No6_Syn_HAY_Fig5-150x150.jpg) |
Figure 5: (2E,6E)-2,6-bis({3-[(2,6-dimethylmorpholin-4-yl)methyl]-4-hydroxy-5-methoxy phenyl}methylidene)cyclohexan-1-one (2d): Click here to View figure |
![(2E,6E)-2,6-bis({4-hydroxy-3-methoxy-5- [(4-methylpiperazin-1-yl)methyl] phenyl}methylidene) cyclohexan-1-one (2e):](http://www.orientjchem.org/wp-content/uploads/2017/12/Vol32_No6_Syn_HAY_Fig6-150x150.jpg) |
Figure 6 : (2E,6E)-2,6-bis({4-hydroxy-3-methoxy-5-[(4-methylpiperazin-1-yl)methyl] phenyl}methylidene) cyclohexan-1-one (2e):
|
Conclusion
A series of di-Mannich bases of cyclovalone derivatives were synthesized and their free radical-scavenging activity evaluated. The data obtained demonstrated the effect of the basicity of Mannich substituent of the compound on the antioxidant activity. The di-Mannich derivative of cyclovalone with diethylamine and dimethylamine (2a and 2b) exhibited higher free radical-scavenging activity than 1.
Acknowledgements
The authors thank the Directorate of Research and Community Service Universitas Indonesia, Depok, Indonesia, for the financial support of this research, and the Department of Chemistry, Faculty of Natural Sciences, Bandung Institute of Technology (ITB), Bandung, Indonesia, for recording NMR and HRMS spectral data.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Young, I. S.; Woodside, J. V. J. Clin. Pathol. 2001, 54, 176-186.
CrossRef - Roman, G. Eur. J. Med. Chem. 2015, 89, 743-816.
CrossRef - Dontha, S. Asian J. Pharm. Clin. Res. 2016, 9, Suppl. 2, 14-32.
- Bayomi, S. M.; El-Kashef, H. A.; El-Ashmawy, M. B.; Nasr, M. N.; El-Sherbeny, M. A.; Badria, F. A.; Abou-zeid, L. A.; Ghaly, M. A.; Abdel-Aziz, N. I. Med. Chem. Res. 2013, 22, 1147–1162
CrossRef - Sardjiman, S. S.; Reksohadiprodjol, M. S.; Hakim, L.; van der Goot, H.; Timmerman, H. Eur. J. Med. Chem. 1997, 32, 625-630
CrossRef - Nurrochmad, A.; Hakim, A. R.; Margono, S. A.; Sardjiman; Yuniarti, N. Int. J. Pharmacy Pharm. Sci. 2010, 2 (3), 45-48.
- Yerdelen, K. O.; Gul, H. I.; Sakagami, H.; Umemura, N. J Enzyme Inhib. Med. Chem., , 2014, Early Online, 1-6.
- tokawa, H.; Shi, Q.; Akiyama, T., Morris-Natschke, S. L.; Lee, K. H. Chinese Medicine, 2008, 1-13.
- Reddy, M. V. B.; Su, C. R.; Chiou, W. F.; Liu, Y. N.; Chen, R. Y.; Bastow, K. F.; Lee, K. H.; Wu, T. S. Bioorg. & Med. Chem. 2008, 16, 7358–7370.
CrossRef - Subramaniapillai, S. G. J. Chem. Sci. 2013, 125 (3), 467–482.
CrossRef - Liu, R.; Zhao, B.; Wang, B. E.; Yao, T.; Pang, L.; Tu, Q.; Ahmed, S. M.; Liu, J. J.; Wang, J. Molecules, 2012, 17, 14748-14764
CrossRef - Bandgar, B. P. ; Patil, S. A. ; Gacche, R. N. ; Korbad, B. L.; Hote, B. S. ; Kinkar, S. N.;Jalde, S. S. Bioorg. Med. Chem. Lett. 2010, 20, 730-733.
CrossRef - Bala, S.; Sharma, N.; Kajal, A.; Kamboj, S.; Saini, V. Int. J. Med. Chem. 2014, 1-15.
CrossRef - Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed., 2005, John Wiley & Sons, Inc.: New York, NY, USA.
- Dank, C.; Felsinger, S.; Kirchknopf, B.; Mastalir, M.; Kählig, H.; Roller, A.; Arion, V. B.; Gstach, H. Molecules, 2015, 20, 1686-1711
CrossRef - Chemaxon Ltd. http://www.chemaxon.com
- Brand-Williams W.; Cuvelier M. E.; Berset C. Lebenson Wiss Technol. 1995, 28, 25-30.
CrossRef - Kedare, S. B.; Singh. R. P. J. Food Sci. Technol. 2011, 48 (4), 412–422
CrossRef - Brewer, M. S. Compr. Rev. Food Sci. Food Safety, 2011,10(4), 221–247
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









