Synthesis and Characterization of Biodegradable Film Chitosan and Carboxymethyl Chitosan to Substitute Silver Wound Healer Plaster
Amri Setyawati1 , Indriana Kartini2, Deni Pranowo2 Dan Lisna Junaeni Muiz3 and Syafitri Hasyati2
, Indriana Kartini2, Deni Pranowo2 Dan Lisna Junaeni Muiz3 and Syafitri Hasyati2
1Chemistry Study Program, Islamic University of Indonesia, Yogyakarta, Indonesia
2Chemistry Department, Universitas Gadjah Mada, Yogyakarta, Indonesia.
3Chemistry Department, Universitas Matha’ul Anwar, Banten, Indonesia.
Corresponding author Email: amrisetyawati@uii.ac.id
DOI : http://dx.doi.org/10.13005/ojc/330637
Currently, wound dressing is made of fabric or polyvinyl chloride (PVC) plastics with silver as active ingredients. Residues of silver can be toxic in the environment and PVC waste is a very long decomposed by soil. Chitosan and carboxymethyl chitosan (CMC) film have been synthesized as a biodegradable material that potential to replace silver wound plasters. CMC film has been synthesized from chitosan with a wide range of molecular weight. Chitosan with high molecular weight resulting from the reflux (1a) while the low molecular weight chitosan produced from microwaves assisted organic synthesis-MAOS (1b). All chitosan and CMC are then molded into a film chitosan and CMC and their properties were analyzed by solubility test. The COOH number of the CMC is also measured by titration. The results show that the CMC were synthesized from chitosan MAOS (2b) has higher COOH group than CMC from chitosan reflux (2a). Chitosan with MAOS process is able to produce a more homogeneous and has a lower molecular weight in a short time. Chitosan 1a films are strong and rigid while chitosan films 1b and CMC films have fragile nature. Test of solubility in physiological solution showed that the CMC films lost 78.56% Massa while chitosan films 1a only 44.59%. This show that a combination of chitosan films for wound plaster with CMC films as a wound healer active ingredient is suitable for replacing the fabric wound plasters contains silver as an active ingredient.
KEYWORDS:chitosan; carboxymethyl chitosan; film; wound healer
Download this article as:| Copy the following to cite this article: Setyawati A, Kartini I, Pranowo D, Muiz D. L. J, Hasyati S. Synthesis and Characterization of Biodegradable Film Chitosan and Carboxymethyl Chitosan to Substitute Silver Wound Healer Plaster. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Setyawati A, Kartini I, Pranowo D, Muiz D. L. J, Hasyati S. Synthesis and Characterization of Biodegradable Film Chitosan and Carboxymethyl Chitosan to Substitute Silver Wound Healer Plaster. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40720 |
Introduction
A skin injury is generally requires cover from being infected by bacteria or pathogens abundant in dust and air12. In fact, many types of bacteria that live on human skin can make infection3. Currently, the plaster dressing’s products made from PVC material that is difficult to decompose with the silver embedded fabric as an active ingredient and material polyvinyl chloride used as an adhesive. Residues of silver can be toxic in the environment. PVC, fabrics and plastics plaster can also pollute the soil because it takes a long time to degrade.
Chitosan and carboxymethyl chitosan is reported as a potential wound healer polymer3,4 and also biodegradable. Substitution of toxic active ingredients such as silver with chitosan and its derivatives are expected to reduce environmental pollution. If this material developed into wound plaster, its ability to inhibit bacterial growth can prevent infection in wounds.
One of the chitosan derivatives is carboxymethyl chitosan. Chitosan is a non-soluble compound in water and only soluble in certain organic solvents in acid conditions56. This led to the limitation use of chitosan to be directly applied. Otherwise, carboxymethyl chitosan is water-soluble and showed lower toxicity than chitosan. Carboxymethyl chitosan is a chemical compound that is more stable, water soluble, biodegradable, biocompatible, and non-toxic than unmodified chitosan78. Carboxymethyl chitosan also has more active site than chitosan that can be more potential as antibacterial agents and pathogens9.
Anti-microbial activity of chitosan and carboxymethyl chitosan make this compound widely applied in agricultures to inhibit the growth of fungi and bacteria during storage of vegetables and fruits10. Because of these polymers have a very low toxicity and safe if absorbed body, this application is widely used, especially in the food and medical industries. Conversely, chitosan is not widely used as anti-microbial agents in the food industry and health because it can change the color and smell10. In addition, carboxymethyl chitosan can also be applied to the environment that is made as membranes or adsorbent for wastewater treatment11. Other applications of carboxymethyl chitosan in the medicals and pharmaceuticals are used to control the release of drug compounds, wound healing and nerve repair cartilage1213.
Carboxymethyl chitosan effects as a wound healing agent are reported by some researcher. Carboxymethyl chitosan can stimulate the proliferation9 and collagen secretion of normal skin and keloid fibroblasts1415. This is very useful for accelerating wound healer. A water-soluble derivative of chitin and chitosan even reported improving skin sores, nerve, cartilage, and bones1617.
Some researchers have reported the benefits of chitosan for various purposes including wound repair3,18–20. Various methods of organic synthesis have also been widely reported. But the relations of molecular weight to the number of COOH group’s and bio-solubility of CMC no one has studied. Whereas the number of COOH groups should be very important to investigate because it deals with anti-bacterial properties owned chitosan and its derivatives. In this present study, it has been conducted a synthesis of chitosan with reflux and microwave radiation technique which is then synthesized into CMC and molded into the plaster. Then the relation of material chitosan with a number of COOH formed is observed, also it solubility in physiological solution and their physical appearance.
Materials and Methods
All material used is from E.Merck with pro analysis (p.a) quality i.e mono chloroacetate, potassium hydroxide, isopropanol, ethanol, and calcium acetate. Infrared spectra were recorded with a Shimadzu Prestige-21 FTIR spectrometer using KBr disc.
Synthesis of CMC
This process is done by the direct alkylating methods wich is N, O-Carboxymethyl chitosan as the target molecule. About 0.5 g chitosan powder with various molecular weights from reflux and MAOS at various watt and time was added by 11 mL isopropanol in Beaker glass and stirred for 20 minutes. After that, please added 4 mL NaOH 40% b/v drop wise at 50°C, and followed by 1 M mono chloroacetate in isopropanol. Stand the reaction in stirrer condition at 50°C for 20 hours and then end with adding the reaction mixture with ethanol 70%. The precipitated product was filtered, washed with ethanol and dried in an oven at 45°C.
Determine the carboxylic acid groups
An amount of 100 mg carboxymethyl chitosan in the Erlenmeyer flask was added by 10 mL calcium acetate 0.5 M and 40 mL distilled water. The blank standard also prepares by this solution without carboxymethyl chitosan. The sample and standard solution were shaken for 24 hours, and then the precipitate formed was filtered and washed with 10 mL distilled water. The filtrate is collected and then titrated with 0.1 M NaOH solution with pp indicator until the color changes to pink. The results obtained are then calculated by the equation proposed by Stevenson (1994).
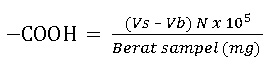
Vs and Vb are the volumes of a standard alkaline solution used for titrating the sample solution and the blank, and N is the normality of a standard alkaline solution.
ilm forming and solubility test
A total of 1% (w/v) chitosan from reflux and microwave was dissolved in a solution of acetic acid 1% v/v was placed in a petri dish. It is left to dry at 60°C to form a thin layer. Then, 3 M of NaOH is added in to be able to remove the thin layer from the petri dish. A thin layer is then washed with distilled water until neutral and wind dried. Meanwhile, to make a thin layer of carboxymethyl chitosan does not need to be dissolved in an acid solution. One percent of carboxymethyl chitosan solution (w/v) in distilled water were placed in a polystyrene petri dish and then allowed to dry at 60°C.
Solubility test of chitosan and carboxymethyl chitosan films is done by the method Wongpanit et al (2011). The film with a particular initial weight, soaked in a solution of physiological 0.1% NaCl w / v for 48 hours at 37 ° C. the solubility of the film is calculated from the amount of weight lost after soaking according to the equation 3:10.
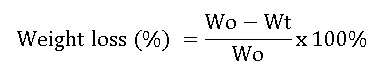
Wo = initial weight before immersion and after immersion; Wt = weight
Results and Discussion
There are two methods of synthesis of carboxymethyl chitosan i.e. a reductive alkylation and alkylation directly21. In this study, carboxymethyl chitosan was synthesized by direct alkylation methods. In the reductive alkylation method, -NH2 of chitosan unit reacts with the carbonyl group of the aldehyde-glyoxylic acid and hydrogenated by NaBH4 or NaCNBH3 to form N-carboxymethyl chitosan. With this method clearly carboxymethyl substituent will be attached to the N atom and almost nothing is attached to the atom O. While direct alkylation methods using mono haloacetate to create derivative N-carboxymethyl chitosan and O-carboxymethyl chitosan or mixed with some of the reaction conditions.
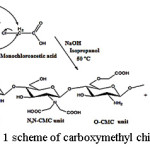 |
Figure 1: scheme of carboxymethyl chitosan synthesis Click here to View figure |
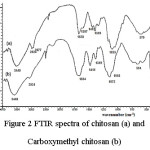 |
Figure 2: FTIR spectra of chitosan (a) and Carboxymethyl chitosan (b) Click here to View figure |
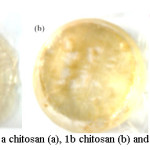 |
Figure 3: Film forming of 1a chitosan (a), 1b chitosan (b) and Carboxymethyl chitosan (c) Click here to View figure |
The purpose of this synthesis of carboxymethyl chitosan is making as many carboxylate substituents included in the structure of chitosan. The more acetate substituent is attached; the carboxymethyl chitosan produced more water soluble and has more anion -COO– to inhibit the growth of gram-positive bacterium Staphylococcus aureus which is widely presented on the wound. Therefore, the method chosen in this study is the method of direct alkylation.
Chitosan powder with various molecular weights from reflux and MAOS at various watt and time then called by chitosan 1a, 1b, 1c and 1d respectively. A modification of chitosan into CMC is characterized by FTIR and COOH group measurement. The results of the FTIR spectra are presented in Figure 2. Figure 2 showed absorption peaks at 1700 cm-1 region which is the vibration of a carbonyl group CO, accompanied by increased absorption peaks in the 1000-1150 cm-1 which are the CO stretching vibration indicates an increase in the ratio of the number of groups than other groups in the molecule or can be said to be an acetyl group (-CH2COOH) has been included in the structure of carboxymethyl chitosan to form chitosan.
The results of COOH groups are presented in Table 1. The data in Table 1 also shows that the number of the COOH increases with decreasing the molecular weight of chitosan. The Smaller and more amorphous polymer chitosan are more effective to make CMC. The COOH property is very important for this film because COOH group has ability against bacteria. The more COOH groups formed of this polymer, it is to be expected that the film can protect the skin from infection effectively.
Tabel 1: Effect of chitosan MW to the number of COOH groups formed
| Chitosan resources | Power (Watt) | MW(g/mol) | Labeling | COOH number |
| Reflux | 1035 | 6051 | 1a | 56,580 cmol/kg |
| Microwave | 400 | 4255 | 1b | 70,000 cmol/kg |
| Microwave | 560 | 4492 | 1c | 6,110 cmol/kg |
| Microwave | 800 | 3794 | 1d | 82,950 cmol/kg |
The addition of microwave radiation time will lower the molecular weight of chitosan 22. Chitosan and carboxymethyl chitosan with a smaller molecular weight tend to have smaller polymer size23. If the size of the polymer decreases the rigidity and steric hindrance also declined. Synthesize of chitosan by microwave radiation tend to form an amorphous solid that is easier to soften by NaOH into a gel. Consequently, more monomers are activated chitosan becomes nucleophile ready to attack mono chloroacetic acid. The amorphous layer of chitosan will also be more easily penetrated by mono chloroacetic acid, so the reaction can be more effective to produce carboxymethyl substitution on chitosan.
Figure 3 show the physical properties of chitosan and CMC film. Chitosan film from reflux (a) is strong and rigid while chitosan films from MAOS (b) and CMC films (c) have fragile nature. Strangeness and flexibility of chitosan are needed for the substitution of plastic PVC, while the bio-solubility is the most importance properties for CMC as the active agent. Test of solubility in physiological solution (1% NaCl) showed that the CMC films lost 78.56% it’s mass while chitosan film is only 44.59%. This is because the film carboxymethyl chitosan is much more soluble and degraded in physiological solution than chitosan films. Carboxymethyl chitosan has a lot of groups that can form anions (-COO-) and even can also cations (NHx+) when forming a mixture of N/O/N, N/N, O-carboxymethyl chitosan, and thus more easily soluble in physiological solution in which the main components is water. This show that a combination of chitosan films for wound plaster with CMC films as a wound healer active ingredient is suitable for replacing the fabric wound plasters contains silver as an active ingredient.
Conclusion
The lower molecular weight of chitosan is good for the synthesis of carboxymethyl chitosan with a higher amount of COOH groups. Carboxymethylchitosan film has better bio-solubility than chitosan film that makes these films suitable to be applied as a combination plaster wounds.
Acknowledgement
We thank the ministry of higher education of Indonesia (DIKTI) for funding this research via PKM-P program 2012.
References
- Moore, L.S.P.; Freeman, R.; Gilchrist, M.J.; Gharbi, M.; Brannigan, E.T.; Donaldson, H.; Livermore, D.M.; Holmes, A.H.; J. Antimicrob. Chemother. 2014, 69, 3409–3422.
CrossRef - James, R.; Upjohn, L.; Cotta, M.; Luu, S.; Marshall, C.; Buising, K.; Thursky, K.; J. Antimicrob. Chemother. 2015, 26, 1912–1918.
- Parvez, S.; Rahman, M.; Khan, M.; Khan, M.A.; Islam, M.J.; Ahmed, M.; Rahman, M.F.; Ahmed, B.; Polym. Bull. 2012, 69, 715–731.
CrossRef - Yan, X.; Guang, X.; Wu, Z.; Jin, H.; Cha, D.; Carbohydr. Polym. 2011, 83, 1479–1485
CrossRef - Shepherd, R.; Reader, S.; Falshaw, A.; Glycoconjugate Journal. 1997, 14: 535-542.
CrossRef - Wang, L.; Li, Q.; Wang, A.; Polym. Bull. 2010, 65, 961–975.
CrossRef - Wang, L.; Chen, X.; Xu, Q.; Zhong, D.; J Mater Sci Mater Med. 2007, 18, 1125–1133.
CrossRef - Fan, L.; Wang, L.; Gao, S.; Wu, P.; Li, M.; Xie, W.; Liu, S.; Wang, W.; Carbohydr. Polym. 2011, 86, 1167–1174.
CrossRef - Feng, C.; Chen, X.; Zhang, J.; Sun, G.; Cheng, X.; Wang, Z.; Park, H.; Front. Chem. China. 2011, 6, 31–37.
CrossRef - Kong, M.; Guang, X.; Xing, K.; Jin, H.; Int. J. Food Microbiol. 2010, 144, 51–63.
CrossRef - Zhao, Z.; Wang, Z.; Wang, S.; Desalination 2002, 144, 35–39.
CrossRef - Muzzarelli, R. A. A. Carbohydr. Polym. 2009, 76, 167–182.
CrossRef - Hawary, D. L.; Motaleb, M. A.; J radioanal Nucl Chem. 2011.
- Janvikul, W.; Uppanan, P.; Thavornyutikarn, B.; Prateepasen, R.; Swasdison, S.; J Mater Sci Mater Med. 2007, 18, 943–949.
CrossRef - Chen, X.; Wang, Z.; Liu, W.; Park, H.; Biomaterials. 2002, 23, 4609–4614 (2002).
CrossRef - Tunney, M. M.; Brady, A. J.; Buchanan, F.; Newe, C.; Dunne, N. J.; J Mater Sci Mater Med 2008, 19, 1609–1615.
CrossRef - Stone, C. A.; Wright, H.; Clarke, T.; Powell, R.; Devaraj, V. S.; Br. J. Plast. Surg. 2000, 53, 601–606.
CrossRef - Shuya, H.; Huang, S.; Han, B.; Shao, K.; J. Ocean Univ. China. 2014, 13, 837–838.
CrossRef - Sikai, P.; Wanshun, L.; Baoqin, H.; Jing, C.; Minyu, L.; Xuan, Z.; J. Ocean Univ. China. 2011, 10, 369–370.
CrossRef - Jannah, N.; Sebri, M.; Anuar, K.; Amin, M. A. T.; OJC. 2017, 33, 628-636.
- Mourya, V. K.; Inamdar, N. N.; Tiwari, A.; Adv. Mat. Lett. 2010, 1, 11–33.
CrossRef - Sahu, A.; Goswami, P.; Bora, U.; J Mater Sci: Mater Med. 2009, 20, 171–175.
CrossRef - Tan, Y.; Han, F.; Ma, S.; Yu, W.; Polym. 2011, 84, 1365–1370.

This work is licensed under a Creative Commons Attribution 4.0 International License.









