Kinetic Evaluation of Influence of Surfactant on Oxidation Reaction of Carboxylic Acid by Molecular Hmn O4
Vandana Sharma1, Kirti Yadav2 P. C. Choyal3 and V. R. Chourey4
1Department of Chemistry Govt. Holkar Science Autonomous College Indore, India.
2Kashutrba Gram Rural College, Indore, India.
3Govt. P.G. College, Dhar, India.
4Govt. Holkar Science Autonomous College Indore, India.
Corresponding author Email: vandana203@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330662
Surfactant catalyzed oxidation of n-butyric acid has been carried out for kinetic and mechanistic investigations in sulphuric acid medium by molecular HMnO4 species. Reaction has also been observed in absence of surfactant.The substrate oxidised in two steps in which first step is slow in comparision to second step which fast one. Micelles of surfactant work as positive catalyst. Substrate and oxidant both follows first order kinetics.Different hypotheses have been applied related to the acidity function to arrive the correct reaction mechanism. Number of moles between substrate and oxidant has also been determined. Temperature variation parameters presented in terms of activation parameters, those are found in prescribed limit. Mechanism for reaction has been suggest on the basis of parameters observed.
KEYWORDS:Kinetics; Micelles surfactant; Oxidation; acidic permanganate ion
Download this article as:| Copy the following to cite this article: Sharma V, Yadav K, Choyal P. C, Chourey V. R. Kinetic Evaluation of Influence of Surfactant on Oxidation Reaction of Carboxylic Acid by Molecular HmnO4. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Sharma V, Yadav K, Choyal P. C, Chourey V. R. Kinetic Evaluation of Influence of Surfactant on Oxidation Reaction of Carboxylic Acid by Molecular HmnO4. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40540 |
Introduction
Now a day’s surfactant has been involved in solution kinetics due to their catalytic efficiency at Pre CMC level. Monomers of surfactant form longer aggregates those are known as micelles1-7. In present work an anionic surfactant i.e. sodium lauryl sulphate is used as miceller catalyst. The oxidation kinetics of carboxylic acids has become one of the very interesting subject of investigation and have been examined by number of workers. Since little work has been reported on the oxidation of carboxylic acid by HMnO4 species in presence of micelles as catalyst and about the kinetics and mechanism of many of its important reactions8-12 hence the present work has been taken for the research. The oxidation has also been carried out by potassium dichromate from the comparative point of view13-16.
The role of permanganate ions as an analytical oxidizing agent in synthetic and analytical works is well known hence it is selected as oxidant in the present investigation. Attempts have also been made to elucidate the type of reaction mechanism which is based on the influence of H+ ion on the rate of oxidation reaction. The Zucker-Hammett, Bunnett and Bunnett-Olsen hypotheses were applied to know the role of water molecule in the reaction mechanism.
Materials and Method
Kinetic studies were performed on Systronic 104 spectrophotometer at 525nm i.e. at the absorbance maximum of permanganate at constant temperature and at 420nm i.e. at the λmax of dichromate. It was verified that there is no interference from other reagent at these wavelengths. All kinetic measurement was conducted under pseudo first order conditions where the substrate was maintained in a large excess over the permanganate and dichromate ion concentration.
The standard solutions of n-butyric acid (BDH) and sodium lauryl sulphate (BDH) were prepared in doubly distilled water. H2SO4 (Analar) was used as a source of hydrogen ion. Permanganate solution was prepared as given by Vogel17. Potassium dichromate solution of Analar grade from Rankem was used.
Result
Dependency of reaction rate on permanganate concentration and potassium dichromate
Dependency of reaction rate on permanganate has been studied by varying is initial concentration at constant concentration of other reactants. It shows that rate constant does not alter with variation in oxidant concentration. The plot of log of optical density i.e. log(a-x) and time exhibit double stage process each being linear. It confirms first order kinetics with respect to permanganate (figure-1.1).
The oxidation has also been carried out by potassium dichromate from the comparative point of view. In this case it has been observed that the reaction is single stage process (figure-1.2).
From the comparative point of view it has been confirmed that oxidation by dichromate is possible at very low concentration of sulphuric acid in comparision to the oxidation by permanganate. It has also been observed that the rate of oxidation by dichromate is comparatively fast than by permanganate.
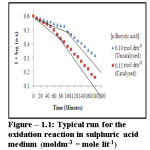 |
Figure 1.1: Typical run for the oxidation reaction in sulphuric acid medium (moldm-3 = mole lit-1) Click here to View figure |
![Figure - 1.2: variation of [Dichromate]](http://www.orientjchem.org/wp-content/uploads/2017/12/Vol32_No6_Kin_Van_Fig2-150x150.jpg) |
Figure 1.2: variation of [Dichromate] Click here to View figure |
Study of variation of substrate concentration
To study the variation of substrate concentration experiments were performed at 308 K with different initial concentration of substrate, other reactants concentration were kept constant. The rate for both in uncatalyzed and catalyzed reaction was found to increase with the concentration of substrate. Table 1.
Table 1 :Variation of n-butyric acid concentration (Uncatalyzed and Catalyzed)
|
[H2SO4] = 4.0 mole lit-1 Temperature = 308K |
[KMnO4] = 1.0×10-3 mole lit-1 [NaLS] = 1.0×10-4 mole lit-1 |
||||||||
|
|
Uncatalyzed |
Catalyzed |
|||||||
|
n-butyric acid mole lit-1 |
× 10-3Min-1 |
× 10-3Min-1 |
× 10-3Min-1 |
× 10-3Min-1 |
|||||
|
|
By Differential method |
By Graphical method |
By Differential method |
By Graphical method |
By Differential method |
By Graphical method |
By Differential method |
By Graphical method |
|
|
0.05 |
1.15 |
1.22 |
2.81 |
2.92 |
1.26 |
1.28 |
2.46 |
2.53 |
|
|
0.10 |
2.07 |
2.01 |
5.10 |
5.06 |
2.32 |
2.28 |
4.42 |
4.28 |
|
|
0.15 |
3.12 |
3.06 |
7.50 |
7.45 |
3.53 |
3.54 |
6.48 |
6.47 |
|
|
0.20 |
4.03 |
4.10 |
10.12 |
9.96 |
4.61 |
4.72 |
8.67 |
8.65 |
|
|
0.25 |
5.00 |
4.95 |
12.40 |
11.36 |
5.69 |
5.64 |
11.06 |
11.11 |
|
|
0.30 |
5.80 |
5.53 |
15.08 |
13.94 |
6.39 |
6.33 |
12.51 |
11.74 |
|
![Figure -1.3: log k against log [Substrate] Catalysed](http://www.orientjchem.org/wp-content/uploads/2017/12/Vol32_No6_Kin_Van_Fig3-150x150.jpg) |
Figure 1.3: log k against log [Substrate] Catalysed Click here to View figure |
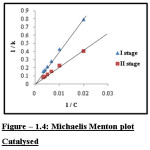 |
Figure 1.4: Michaelis Menton plot Catalysed Click here to View figure |
Effect of the variation of the [H+] concentration
The reaction was studied at different sulphuric acid concentrations, keeping the constant concentration of n-butyric acid, potassium permanganate and sodium lauryl sulphate. The variation of the [H+] concentration on the reaction rate has been investigated. The experimental results obtained are presented in table 2.
|
[n-butyric acid] = 0.20 mole lit-1 Temperature = 308K |
[KMnO4] =1.0 × 10-3 mole lit-1 [NaLS] = 1.0 ×10-4 mole lit-1 |
||||
|
[ H+] mole lit-1 |
-H0 |
×10-3 Min-1 |
×10-3 Min-1 |
||
|
2.0 |
0.84 |
0.043 |
1.20 |
2.41 |
|
|
2.5 |
1.12 |
0.063 |
1.33 |
3.25 |
|
|
3.0 |
1.38 |
0.085 |
1.63 |
4.75 |
|
|
3.5 |
1.62 |
0.111 |
2.04 |
6.20 |
|
|
4.0 |
1.85 |
0.142 |
2.44 |
8.67 |
|
Applicability of Zucker-Hammett, Bunnett and Bunnett-Olsen hypotheses
|
[n-butyric acid] = 0.20 mole lit-1 Temperature = 308K |
[KMnO4] = 1.0 × 10-3 mole lit-1 [NaLS] = 1.0 ×10-4 mole lit-1 |
|||
|
S.No |
Correlation |
Parameters |
Slope value (H2SO4 medium) |
|
|
I stage |
II stage |
|||
|
1. |
Zucker-Hammett plot |
|||
|
(a) |
(log Rate constant) against -H0 |
Slope |
0.316 |
0.553 |
|
(b) |
(log Rate constant) against log [H+] |
Slope |
1.23 |
1.58 |
|
2. |
Bunnett’s plots |
|||
|
(a) |
(log Rate constant + H0) against |
(ω) |
-6.88 |
-4.51 |
|
(b) |
(log Rate constant – log [H+]) against |
(ω*) |
0.250 |
2.62 |
|
(c) |
(log Rate constant] – log [H+] –H0) against |
Slope |
10.37 |
12.74 |
|
3. |
Bunnett – Olsen plot (L.F.E.R) |
|||
|
(a) |
(log Rate constant +H0) against (H0 + log [H+]) |
(ф ) |
0.972 |
0.636 |
NaLS concentration variation
Effect of variation of concentration sodium lauryl sulphate (NaLS) has been studied keeping the other reactants constant at given constant temperature.
It is clear that the pseudo first order rate constant for the given concentration range of NaLS increases with the increase in NaLS Concentration (Table 4). In the present study, it has been suggested that the formation of premicellar aggregates is responsible for the miceller catalysis.
The catalysis by the concentration of micelle which is below their cmc (i.e. critical micelles concentration) is termed as premiceller catalysis as reported in the literature 24-26. It has been determined graphically by plotting a Piszkiewicz graph between log [kobs – ko] / [km – kobs] and log [NaLS]. Here ko is the velocity constant in absence of NaLS, kobs is the velocity constant at different NaLS concentration, while km is the maximum velocity constant in presence of NaLS used. The range of variation of NaLS were kept between from 5.0×10-4 to 9.0 × 10-4 mol liter-1. Its maximum concentration used is 9.0 × 10-4 mole lit-1. This is well below the reported cmc of sodium lauryl sulphate as reported in the literature is 8.1× 10-3 mole lit-1 at 298K. This confirms that the observed phenomenon is a case of premiceller catalysis.
The slope value ‘n’ obtained from this graph which is a good straight line for first and second stages are 1.86 and 2.01.These are between the expected value i.e. between 1 to 6 which is for premiceller catalysis. In the case of miceller catalysis this value should be more than 20. This is according to the binding parameters as suggested by Piszkiewicz27.
The results have been reported in the table 4 for both the stages. The ‘n’ value of positive cooperativity also supports the substrate promoted micellization which is analogous to positive cooperativity in case of enzymatic reactions28-30.
|
[n-butyric acid] = 0.10 mole lit-1 [H2SO4] = 4.0 mole lit-1 |
[KMnO4] = 1.0 × 10-3 mole lit-1 Temperature = 308K |
||||
|
[NaLS] mole lit-1 |
×10-3 Min-1 |
×103 litre mol-1Min-1 |
×103 Min-1 |
×103 litre mol-1 Min-1 |
|
|
Uncatalyzed(Absent) |
2.10 |
—- |
3.99 |
—- |
|
|
1.0 × 10-4 |
2.32 |
2.20 |
4.41 |
4.20 |
|
|
2.0 × 10-4 |
2.58 |
2.40 |
4.81 |
4.10 |
|
|
3.0 × 10-4 |
2.86 |
2.53 |
5.30 |
4.36 |
|
|
4.0 × 10-4 |
3.04 |
2.35 |
5.70 |
4.27 |
|
|
5.0 × 10-4 |
3.29 |
2.38 |
6.15 |
4.32 |
|
|
6.0 × 10-4 |
3.59 |
2.48 |
6.50 |
4.18 |
|
Change in Temperature on reaction
The reaction was studied at different temperature i.e. 303, 308, 313 and 318K at the constant concentration of all the reactants. The result has been presented in the table 5.
Table 5: Effect of Temperature variation
|
[n-butyric acid] = 0.10 mole lit-1 [H2SO4] = 4.0 mole lit-1 |
[KMnO4] = 1.0 × 10-3 mole lit-1 Temperature = 308 K |
||||
|
Temperature (K) |
Velocity constant for Uncatalyzed NaLS = Nil |
Velocity constant for Catalyzed NaLS = 1.0× 10-4 mole lit-1 |
|||
|
|
×10-3 Min-1 |
×10-3 Min-1 |
×10-3 Min-1 |
×10-3 Min-1 |
|
|
303 |
1.38 |
2.10 |
1.84 |
2.44 |
|
|
308 |
2.10 |
3.99 |
2.32 |
4.41 |
|
|
313 |
3.10 |
4.50 |
3.83 |
5.07 |
|
|
318 |
4.61 |
8.44 |
4.72 |
8.80 |
|
|
Selected Temperature (K) |
Temp. coeff. for Uncatalyzed |
Temp. coeff. for Catalyzed |
|||
|
|
I step |
II step |
I step |
II step |
|
|
303-313 |
2.24 |
2.14 |
2.08 |
2.07 |
|
|
308-318 |
2.19 |
2.11 |
2.03 |
1.99 |
|
Table 6 : Thermodynamic and Activation Parameters (For Uncatalyzed and Catalyzed reaction)
|
[n-butyric acid] = 0.10 mole lit-1 [H2SO4] = 4.0 mole lit-1 Temperature = 308K |
[KMnO4] = 1.0 × 10-3 mole lit-1 [NaLS]= 1.0 × 10-4 mole lit-1
|
||||||
|
Kinetic Parameters |
|||||||
|
Stages |
∆E≠ KJ mol-1 |
∆H≠ KJ mol-1 |
∆S≠ Jmol-1K-1 |
Frequency factor pZ dm3mol-1 Sec-1 |
∆G≠ KJ mol-1 |
||
|
Cal. |
Gra. |
||||||
|
Uncatalyzed I stage (Without NaLS ) |
63.822 |
64.561 |
62.000 |
-86.83 |
7.78 × 108 |
35.254 |
|
|
Uncatalyzed II stage (Without NaLS ) |
60.102 |
62.301 |
59.741 |
-88.83 |
6.10 × 108 |
32.378 |
|
|
Catalyzed I stage (WithNaLS) |
57.811 |
55.401 |
52.840 |
– 115.75 |
24.05 × 106 |
17.189 |
|
|
Catalyzed II stage (With NaLS) |
57.673 |
58.357 |
55.797 |
– 100.80 |
14.52× 107 |
24.747 |
|
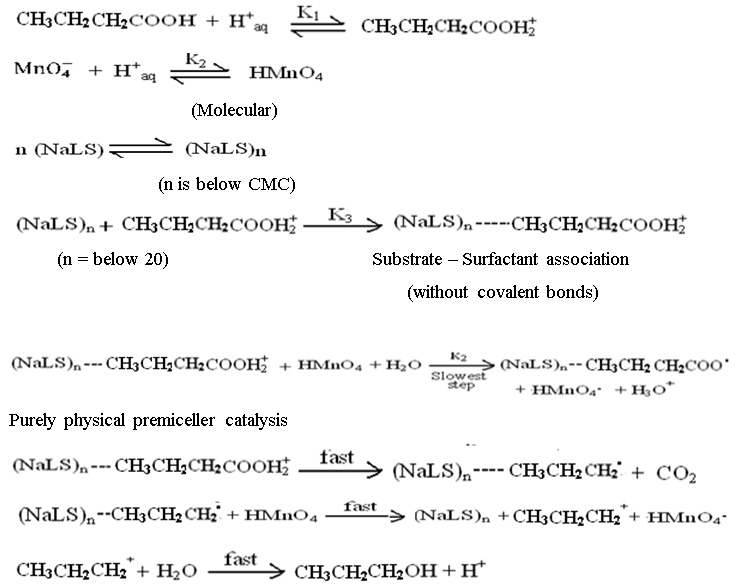
It was determined by analytical method. This involves the known excess of KMnO4 over the substrate i.e. n-butyric acid kept at room temperature for a sufficiently long time (36 hours). The unreacted permanganate was estimated iodometrically. It has been found that the five mole of substrate consumes one mole of potassium permanganate. The catalyzed reaction is suggested to be proceeds as follows:
![]()
Propyl alcohal was identified by spot test method as given by Figel32 and Carbon dioxide have been determined by routine test as the end products. The formation of intermediate free radical is confirmed by induced reduction of mercuric chloride. Low value of energy of activation also supports the same.
Mechanism
On the basis of the results of kinetic measurements facts and discussion the following steps for the oxidation reaction of n-butyric acid in the absence and presence of sodium lauryl sulphate as catalyst has been suggested.
Mechanism and rate law for uncatalyzed oxidation
The mechanism proceeds with the formation of molecular HMnO4 in sulphuric acid medium
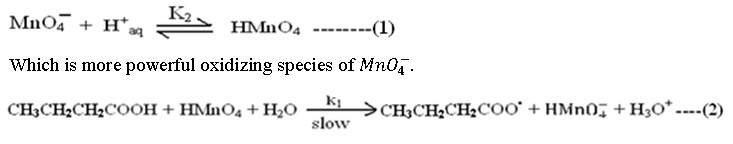
Step (2) is rate determining step. The cause of slowness is the involvement of three body collision which is difficult one. All subsequent steps are very fast and not of kinetic importance, are as follows;
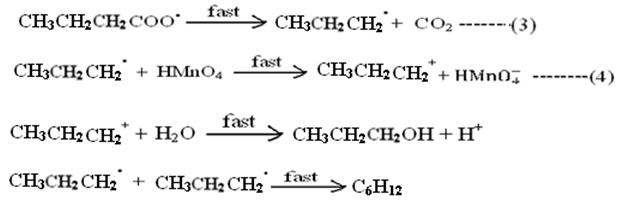
The above mechanism leads to suggest the rate expression for uncatalyzed reaction; in which the concentration of hydrogen ion and water have been withdrawn from main equation.
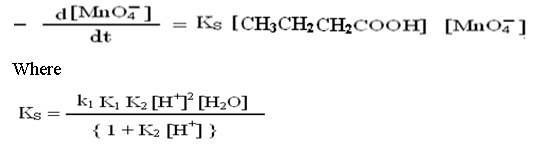
Mechanism and rate law for catalyzed reaction
The reaction rates have been found substantially increased in presence of anionic sodium lauryl sulphate surfactant. It is assumed that due to the electrostatic interaction between n-butyric acid and micelles of surfactant leads the increase in effective concentration which is responsible for the increase in reaction rate. The very low values of number ‘n’ of surfactant molecules i.e. below 20 are expected in micelles. The mechanism proposed, therefore involve the substrate – surfactant interaction, with the premiceller catalysis phenomenon as the n, calculated has the value below 20.
The following rate law expression may be derived from above discussed steps considering that catalyzed and uncatalyzed reactions proceeds simultaneously.
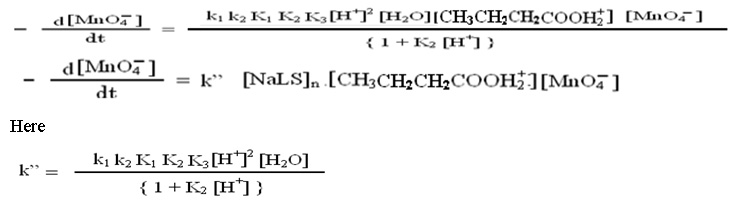
Discussion
In the studied reaction the order for n-butyric acid and molecular permanganate has been found as one i.e. HMnO4 were found one in each case.The role of surfactant is only as premiceller physical catalyst with no covalent bond formation between surfactant – substrate association. Rate law involve the acid concentration term and water molecule. Zucker-Hammett, Bunnett’s and Bunnett-Olsen Hypotheses suggest the involvement of water molecule as a proton abstracting agent in the reaction.
Acknowledgement
The author Vandana Sharma is thankful to Dr. Smt. R. Vyas Head Deptt. of Chemistry Holkar Science College Indore for providing all necessary research facilities.
References
- Bhagwat,V. W.; Pipada, M.;Tumalagada, S. B.; Pare, B. Ind. J. Chem., 2003, 42A, 1644.
- Kabir-ud-din; Shakeel Iqubal, S. M.; Khan, Z. Ind. J. Chem., 2005, 42A,2455.
- Prakash, R. J. Ultra Chem., 2010, 6(3), 357.
- Bende, N.; Chourey, V. R. Res. J. Chem. Environ., 2013, 17(06), 75-87.
- Sharma, V.; Chourey, V. R. J. Ultra Chem., 2013, 09(03), 287-302.
- Sharma, V.; Chourey, V. R. Int. J. Advance Research, 2014, 2(3), 764-772.
- Sharma, V.; Chourey, V. R. Int. J. Advance Research, 2014, 2(6), 617-625.
- Vanangnudi, C.; Subbaiyan, M.; Srinivans, S. Asian J. Chem., 2007, 19(04), 71.
- Hall, L.; Singh, A. C. J. Chem. Educ. 1988, 65,142.
CrossRef - Sharma, A.; Sharma, A. K.; Gupta, K. S. J. Indian Chem. Soc., 2009, 86, 649.
- Wiberg, K. B.; Steward, R. J. Am. Chem. Soc., 1956, 78, 1214.
CrossRef - Bhale, V. M.; Mohammad, S.; Bhagwat, W. V.; Bafna, S. C. J. Sci. Ind. Research (India), 1953, 1954,12,13,521, Chem. Abst., 48,5620.
- Khandual, N. C.; Satpathy, K. K.; Nayak, P. L. Indian J. Chem., 1973,11, 770-772.
- Venkatraman R.; Rao S. B. Indian J. Chem., 1972, 10, 165-167.
- Khan Z.; Kabiruddin Indian J. Chem., 2000, 39(A), 552.
- Wankhade, C. B.; Khodakar, S. N.; Patel, N. T. Asian J. Chem., 2006, 18(4), 2538.
- Vogel, A. I. “A text book of quantitative Inorganic analysis” Longman Green, London, 1964, 282.
- Mehrotra, R. N. J. Chem. Soc., 1968, 1123.
- Sankhla, P. S.; Mehrotra, R. N. Indian J. Chem., 1972,10,1081, 2077.
- Zucker, L.; Hammett, L. P. J. Am. Chem. Soc., 1939, 61, 2791.
CrossRef - Bunnett, J. F. J. Am. Chem. Soc., 1961, 83, 4968.
CrossRef - Bunnett, J. F.; Olsen, F. P. Can. J. Chem., 1899, 1917, 1966, 44.
- Paul, M. A.; Long, F. A. Chem. Rev., 1957,57,1.
CrossRef - Bruice, J. C.; Katzhendler, J.; Fedar, L. J. Am. Chem. Soc., 1968, 90,133.
- Sarasan, G. Asian J. Chem., 2003, 15(1), 133.
- Prakash, R.; Agrawal, S. J. Ultra Chem., 2012, 8(3), 309.
- Piszkiewicz, D. J. Am. Chem. Soc., 1977, 99, 1550.
CrossRef - Raghavan. Ind. J. Chem., 1980, 19(A), 322-324.
- Behara, G. B.; Mishra, S. S.; Samantaray Ind. J. Chem., 1982, 21(A),1063-1065.
- Pare, B.; Kaur, P.; Bhagwat, V. W.; fogliani, C. J. Korean Chem. Soc., 2004, 48,195.
CrossRef - Laidler, K. J. “Chemical Kinetics”, Third Edn. Tata Mc. Graw Hill, Bombay, 2004, 39.
- Fiegl, F. “spot test in Organic Analysis”, 1955, 174.

This work is licensed under a Creative Commons Attribution 4.0 International License.









