Influence of Kinetic Variables on Rubber Seed Oil Transesterification Using Bifunctional Catalyst CaO-MgO/SiO2
Kamisah D Pandiangan1 , Wasinton Simanjuntak1, Mita Rilyanti1 Novesar Jamarun2 and Syukri Arief2
, Wasinton Simanjuntak1, Mita Rilyanti1 Novesar Jamarun2 and Syukri Arief2
1Department of Chemistry, University of Lampung, Bandar Lampung, Indonesia.
2Department of Chemistry, University of Andalas University, Padang, Indonesia.
Corresponding authors E-mail: kamisahdelilawati@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330623
Article Received on :
Article Accepted on :
Article Published : 05 Dec 2017
In this study, syntheses of bifunctional catalyst (CaO-MgO/SiO2) with different ratios of CaO: MgO:SiO2 of 1:1:3 and 1:1:5 were carried out by sol-gel method. The catalysts were subjected to calcination temperatures of 500, 600, 700, 800, 900oC for 6 hours at peak temperatures, and then tested as catalyst in a series of transesterification of rubber seed oil without and with the use of coconut oil as co-reactant. Several kinetic variables of transesterification were also investigated. The results revealed that the catalyst with the composition of CaO-MgO/SiO2 1:1:5 and subjected to calcination temperature of 800°C functioned as the most active catalyst. The highest percent of conversion (90%) was resulted from the experiment with the use of 5% catalyst, 50 mL methanol, 10% co-reactant, temperature of 70oC and reaction time of 6 hours.
KEYWORDS:bifunctional catalyst; sol-gel; rubber seed oil; transesterification
Download this article as:| Copy the following to cite this article: Pandiangan K. D, Simanjuntak W, Rilyanti M, Jamarun N, Arief S. Influence of Kinetic Variables on Rubber Seed Oil Transesterification Using Bifunctional Catalyst CaO-MgO/SiO2. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Pandiangan K. D, Simanjuntak W, Rilyanti M, Jamarun N, Arief S. Influence of Kinetic Variables on Rubber Seed Oil Transesterification Using Bifunctional Catalyst CaO-MgO/SiO2. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40503 |
Introduction
Transesterification is the term used to describe the reaction for converting glyceride to simpler corresponding ester and glycerol. The most important application of this type of reaction is production of biodiesel, in which simple alcohols are reacted with glycerides contained in vegetable oils or animal fats. In principle, transesterification reactions can be carried out by non-catalytic or catalytic methods1,2. Non-catalyst transesterification reaction, also known as supercritical method, requires high temperatures and pressures3,4. For these reasons, supercritical method is less feasible for industrial scale5, therefore catalytic method is the most widely applied.
In practice, catalytic production of biodiesel can be conducted using different catalysts, which can be distinguished into base, acid, and enzymes6-9. The use of acid and base catalysts has been widely published both as homogeneous and heterogeneous catalysts. Homogeneous catalysts are strong acids or bases10-14. The main advantages of homogeneous catalyst include simple operating conditions, high activity and relatively short reaction time. With the use of acid catalyst, esterification of free fatty acid and transesterification of glycerides take place simultaneously, which is very usefully for raw materials with high free fatty acid content9,15. Apart from the above advantages, homogeneous catalysts exhibit several drawbacks, such as saponification reaction, difficulty to separate product from the catalyst, the catalysts are mostly corrosive, and cannot be reused8,9. Due to various disadvantages of homogenous catalysts, development and application of heterogeneous catalysts continue to attract growing interest of researchers as well as industrial society. Heterogeneous catalysts are of different phase with the biodiesel, enabling the separation using simple filtration process. Furthermore, heterogeneous catalysts are non-corrosive and offer the opportunity for reusing the catalysts.
At present, many types of chemical compounds have been tested as heterogeneous catalysts, such as the oxides of various metals, nonmetallic oxides, ion exchanges, zeolites, metal complexes, mixture of metal oxides, and supported metal oxides7,9,16-27. One type of heterogeneous catalyst widely used in for biodiesel production is alkaline earth metal based catalyst. This type of catalyst is of particular interest since divalent metal oxides such as CaO, MgO, BaO, SrO exhibit alkaline characteristic, thus, function as solid base catalysts16-20,25,28. Among the alkaline earth metal oxides, CaO is the most promising for several reasons include low price, low solubility in methanol, not toxic, high catalytic activity, and can be obtained from natural materials such as rocks, minerals, crabs, eggshells, as well as mollusks, simply by calcination treatment16,23,28,29. In practice, CaO can be used as a single catalyst, supported catalyst or mixed catalyst (multifunctional catalyst).
Several previous studies have reported the promising results of the use of silica-supported alkaline earth oxides as heterogeneous catalyst in the transesterification of vegetable oils, such as CaO/SiO2 for palm oil26, corn oil30, rubber seed oil24, and MgO/SiO2 for corn oil30 and castor oil22. In previous studies17,18, the use of CaO and MgO was extended by using both of the oxides in a binary catalyst and the catalyst was tested for transesterification reaction of Jatropha curcas oil. In the study it was reported that the activity of binary catalyst is practically similar to that of single oxide catalyst. However, the stability of binary catalyst is much higher than the single oxide, enabling the reuse of the binary system up to six repetitions. Based on the results of this particular previous study, in this current investigation rice husk silica supported bifunctional catalysts (CaO-MgO/SiO2) with different ratios of CaO:MgO:SiO2 of 1:1:3 and 1: 1:5 were synthesized through sol-gel route. The catalysts were then subjected to calcination treatment at temperatures of 500, 600, 700, 800, and 900oC for six hours at peak temperatures.The activity of the catalysts was tested for transesterification of rubber seed oil without and with the use of coconut oil as co-reactant.
Material and Methods
The chemicals used in this study, sodium hydroxide, nitric acid, and n-hexane, are purchased from Aldrich. Magnesium nitrate and methanol used are reagent grade purchased from Merck. Calcium oxide was obtained from Halaban, a local company in the City of Padang, West Sumatera. Rice husk was obtained from local rice milling industry in the City of Bandar Lampung. Before use, the husk was soaked in distilled water overnight, and sinking husk presumed to contain silica in high quantity, was collected, while floating husk was discharged. Rubber seeds were collected from plantation company (Perkebunan PTPN V), South Lampung. The main instruments used in this study are GC-MS QP2010 SE SHIMADZU for identification of transesterification product and Agilent 500 MHz proton H-NMR instrument.
Experimental methods
Extraction of rubber seed oil
Rubber seed oil used was directly extracted from rubber seed collected from the sampling site in the plantation area. Prior to oil extraction, rubber seed kernel was removed from the hull and cut into four pieces. The kernel was oven dried at 115oC for 10 hours, and dried kernel was subjected to extraction process. Extraction of oil was carried out using DL-ZYJ02 oil press machine.
Extraction of rice husk silica
Rice husk silica was obtained using an alkali extraction method, as previously applied31,32. Rice husk silica was extracted using an alkali extraction process, adopting the method previously applied. Typical experiment was carried out by transferring 50 grams of dry husk into a beaker glass contains 500 mL of 5% NaOH solution. The mixture was boiled for 30 minutes, and then allowed to cool to room temperature and left for 24 hours to optimize the release of silica from the husk. The mixture was filtered to separate the filtrate which contains dissolved silica (silica sol), while the residue was discharged.
Preparation of catalyst
The CaO-MgO/SiO2 heterogeneous catalyst preparation was performed adopting the procedure reported in previous study18,19,33. Typically, 15 g of dried silica was transferred into 450 mL of 1.5% NaOH solution, and the mixture was magnetically stirred to dissolve the silica (silica sol). Specified amounts of CaO and Mg(NO3)2.6H2O to obtain the desired compositions were added slowly into the sol under magnetic stirring to ensure homogeneous mixing of the raw materials. The pH of the mixture was adjusted to neutral range for conversion of the sol into gel. The gel was allowed to stand at room temperature for 24 hours then filtered and then dried in an oven at 110 °C for 24 hours to remove water. The dry CaO-MgO/SiO2 was ground into powder and then calcined at temperatures of 500, 600, 700, 800 and 900 oC. The calcination process is carried out with a gradual heating temperature rise of 10 oC/ min and a holding time of six hours at the peak temperature.
Transesterification experiment
Transesterification of rubber seed oil with methanol was performed in a 500 mL round flask equipped with reflux condenser, carried out at a constant temperature of 70 oC for six hours. All ten catalysts were tested in order to obtain the most active catalyst, which was used further to investigate the effect of kinetic variables include the ratio of methanol to rubber seed oil, the amount of catalyst, the transesterification time, and the use of coconut oil as co-reactant.
To evaluate the effect of methanol to oil ratio, a series of experiments was carried out by varying the volume of methanol of 50, 75, 100, 125 and 150 mL, while keeping the volume of rubber seed oil constant at 25 mL. The amounts of catalysts were catalysts performed were 5; 7.5; 10; 12.5; and 15% relative to the mass of the oil. Transesterification was performed at different reaction times of 2, 3, 4, 5, and 6 hours, and the effect of co-reactant was investigated by using different amounts of coconut oil of 10, 20, 30, 40, and 50% relative to the volume of rubber seed oil used.
Analysis of transesterification product
Identification of FAMEs in the transesterification product was conducted with the aid of gas chromathography-mass spectroscopy (GC-MS) technique. The analysis was carried out on GCMS-QP2010 SE SHIMADZU, equipped with 30 m long and 0.32 mm internal diameter HP SMS 30 m column. The instrument was operated in the EI mode at 70 EV using helium as carrier gas and nitrogen as make up gas to obtain the total flow rate of 60 mL/min. Tentative identification of the components in the sample was done by comparing the mass spectra of the component to those published in the MS Library System NIST62, Wiley 7, database. The relative amount of each component was estimated by dividing the peak area of the component with the total peak area of all components identified. Identification of proton types was conducted using Agilent 500 MHz proton H-NMR using console DD2 system, operated at 500 MHz frequency, with helium as carrier gas, type probe 1H, supported by software Linux VnmrJ 4.1 for proton H-NMR.
Results And Discussion
Rubber seed oil
Extraction of rubber seed oil revealed that the oil content of the seed ranges from 40 – 50% as has been reported in previous study24. The oil obtained has transparent and clear appearance with brown color, with the properties as listed in Table 1.
Table 1: Some properties of rubber seed oil used in this study.
| No. | Parameter | Value |
| 1. | pH | 6.2 |
| 2. | Viscosity (mm2/s) | 18.01 |
| 3. | Density (kg/m3) | 0.92 |
| 4. | Acid number (mg KOH/g) | 47.09 |
| 5. | Saponification number (mg KOH/g) | 189.26 |
| 6. | Free fatty acid (%) | 35.57 |
In general, the properties of rubber seed oil extracted in in this study are in agreement with those reported by others. The existence of rubber seed oil as brown oil was also reported34 the value of density, viscosity, free fatty acid content, acid number, and saponification number, observed in this study are practically similar to those reported by others35,36.
Catalytic activity test
To study the activity of CaO-MgO/SiO2, two sets of experiments were conducted. The first set was conducted without the use of co-reactant and the other with the use of coconut oil as co-reactant. The results showed that transesterification of rubber seed oil without the use of co-reactant led to very low reaction yields and saponification reactions. Therefore, further experiments were carried out by adding coconut oil as a co-reactant, with the aim to help initiating the reaction, since coconut oil is known as a vegetable oil than can be converted into biodiesel very easily. The role of coconut oil as co-reactant in enhancing transesterification of other oils has been reported in previous studies, include castor oil MgO/SiO2 catalyst22 and rubber seed oil using CaO/SiO2 catalyst24.
Effect of catalyst compositions and calcination temperatures
The activity of supported heterogeneous catalyst is generally effected by the compositions and the calcination temperatures applied to the catalyst. The roles of these two factors are reflected by the experimental results shown in Figure 1. In general, the catalyst with the ratio of CaO:MgO:SiO2 = 1:1:5 has higher activity than that with the CaO:MgO:SiO2 ratio of 1:1:3. It is also evident that increasing the calcination temperature up to to 800 °C led to quite significant increase in the catalyst activity. For higher temperatures (900 oC) there is a decrease in the ability of catalysts, most likely due to conversion of the catalyst into crystalline phase, reducing the availability of pores to host the reaction. The results in Figure 1 suggested that the best catalyst is CaO-MgO/SiO2 with the composition of 1:1:5 and calcined at temperature of 800 °C. This particular catalyst was used further to study the effect of other variables.
Effect of kinetic variables
Experimental results showing the effect of catalyst amount, reaction time, ratio of methanol to oil, and the amount of co-reactant are compiled in in Figure 2.
Figure 2 shows the influence of some kinetic variables on the yield of the reaction achieved. In general, the variables investigated display quite significant effect. The results presented in Figure 2a show that the highest yield was obtained with the use of 5% catalyst, while the use of larger quantities of catalyst, the tendency of declining yields was observed. This trend of decreased yields with the use of larger amounts of catalyst might be due to leaching of catalyst, primarily the release of CaO which suppressed the reaction, as suggested in previous studies24,25.
The effect of reaction times is clearly demonstrated by the results in Figure 2b. As can be seen, the experiment carried out with reaction time of two hours produced very small amount of product, but led to significant increase in the yields followed the prolongation of reaction times, reaching the yield of 90% with the reaction time of six hours. The need for relatively long reaction time to achieve high yield is most likely associated high free fatty acid content of the rubber seed oil as shown in Table 1. The experimental results presented in Figure 2c, in which the highest yield was obtained with the use of methanol/oil volume ratio of is 2:1 (50 mL methanol and 25 mL of rubber seed oil), and increasing the volumes of methanol tend to result in decreased reaction yields. This pattern is most likely due to dissolution of some of the catalyst with the use of excessive methanol, leading to formation of soap as observed during the experiments with higher methanol to oil ratios. According to Figure 2d, the maximum conversion rate was obtained by the use of 10% co-reactant. The tendency of reduced yields with larger amounts of co-reactant used is probably due to insufficient amount of methanol since the volumes of methanol were the same for all experiments.
The overall results obtained in this study revealed that the highest yield (90%) was achieved using the catalyst with CaO :MgO : SiO2 ration of 1: 1: 5 and calcined at 800 °C, with the use of methanol to oil ratio of 2: 1, reaction time of six hours, and the use of 10% co-reactant.
GC-MS analysis
To identify the chemical components, the reaction products were analyzed using gas chromatography coupled with mass spectrometry (GC-MS) technique. Typical example of the GC chromatogram of the sample is shown in Figure 3.
Figure 3 shows the existence of four separate peaks, suggesting the presence of four chemical components in the sample. With the aid of NIST12 Library System Software, which contains MS data of various reference compounds, the components of the sample was identified and the results are presented in Table 2.
Table 2: Chemical components of the product of rubber seed oil transesterification.
|
Retention time (min) |
Relative percentage (%) |
Compound name |
Chemical formula |
|
36.1 |
11.83 |
Methyl palmitate | C17H34O2 |
|
39.5 |
11.77 |
Methyl linoleate | C19H34O2 |
|
39.6 |
60.80 |
Methyl oleate | C19H36O2 |
|
40.1 |
13.27 |
Methyl stearate | C19H38O2 |
As displayed by the data in Table 2 the transesterification product of rubber seed oil is a mixture of methyl esters with methyl oleate emerges as the main component. This is in accordance with previous reports12,24 describe the existence of oleic acid as the main component of rubber seed oil. The presence of methyl esters suggest that rubber seed oil studied was successfully converted into biodiesel.
1H-NMR analysis
Further analysis of the biodiesel produced was conducted with proton nuclear magnetic resonance (1H-NMR), and the typical example of the spectrum produced is shown in Figure 4.
Figure 4 shows the characteristics of methoxy protons as singlet characterized by a sharp peak at a chemical shift of 3.40 ppm and a peak in the range of 2.24 to 2.31 ppm is a methylene group of β-carbonyl37,38. The protons of triglycerides are characterized by a peak in the region of 4.08 to 4.27 ppm38. The peak at 1.57 ppm associated with methylene proton at b-carbonyl and the peak in the region of 5.21 – 5.27 ppm is the alkene hydrogen37. Other peaks observed in the region of 0.82 to 0.85 ppm represent the protons in the methyl end group, and the sharp multiplets in the region of 1.21-1.26 ppm originate from overlapping of CH2 proton in the methyl ester mixture, representing the presence of unsaturated methyl esters39.
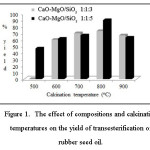 |
Figure 1: The effect of compositions and calcination temperatures on the yield of transesterification of rubber seed oil. Click here to View figure |
 |
Figure 2: The experimental results showing the effect of kinetic variables on the yield of reaction: (a) different amounts of catalyst, (b) different reaction times, (c) different methanol to oil ratios, and (d) different amounts of co-reactant. Click here to View figure |
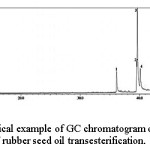 |
Figure 3: Typical example of GC chromatogram of the product of rubber seed oil transesterification. Click here to View figure |
 |
Figure 4: Proton 1H-NMR spectrum of biodiesel produced from rubber seed oil. Click here to View figure |
Conclusion
The CaO-MgO/SiO2 bifunctional catalysts synthesized exhibit catalytic activity for transesterification of rubber seed oil to produce biodiesel. The catalyst with the highest activity is that with the CaO:MgO:SiO2 ratio of 1:1:5 and calcined at 800 °C. Conversion of rubber seed oil into biodiesel are confirmed by GC-MS analysis which shows that the product is a mixture of methyl esters, and with the proton 1H-NMR which demonstrates the agreement with 1H-NMR results the commonly reported for biodiesel.
Acknowledgment
The authors gratefully acknowledge The Directorate of Higher Education, The Ministry of Research, Technology, and Higher Education, Republic of Indonesia, for financial support through research grant, Fundamental Research 2017, contract number: 78/UN26/5/LPPM/2017.
References
- Atabani, A. E.; Silitonga, A. S.; Badruddin, I. A.; Mahlia, T. M. I.; Masjuki, H. H.; Mekhilef, S. Renew. Sust. Energ. Rev. 2012, 16, 2070-2093.
CrossRef - Islam, A.; Taufiq-yap, Y. H.; Chan, E. S.; Moniruzzaman, M.; Islam, S.; Nabi. M. N. Energ. Convers. Manage. 2014, 88, 1200-1218.
CrossRef - Juan, J. C.; Kartika, D. A.; Wu, T. Y.; Hin, T. Y. Y. Bioresource. Technol. 2011, 102, 452-460.
CrossRef - Sawangkeaw, R.; Tejvirat, P.; Ngamcharassrivichai, C.; Ngamprasertsith, S. Energies. 2012, 5, 1062-1080.
CrossRef - Kulkarni, M. G.; Dalai, A. K. Ind. Eng. Chem. Res. 2006, 45, 2901-2913.
CrossRef - Gorji, A.; Ghanei, R. J. Biodivers. Environ. Sci. 2014, 5, 48-59.
- Koh, M. Y.; Ghazi, T .I .M. Renew. Sust. Energ. Rev. 2011, 15, 2240-2251.
CrossRef - Bankovi´c-Ili´c, I. B., Stamenkovi´c, O. S.; Veljkovi´c, V. B. Renew. Sust. Energ. Rev. 2012, 16, 3621-47.
CrossRef - Endalew, A. K.; Kiros, Y.; Zanzi, R. Biomass. Bioenerg. 2011, 35, 3787-3809.
CrossRef - Wong, Y. C.; Devi, S. Orient. J. Chem. 2014, 30, 521-528.
CrossRef - Sivakumar, P.; Anbarasu, K.; Renganathan, S. Fuel. 2011, 90, 147 – 151.
CrossRef - Ahmad, J.; Yusup, S.; Bokhari, A.; Kamil, R. N. M. Energy. Convers. Manage. 2014, 78, 266-275.
CrossRef - Agarwal, M.; Arya, I.; Chaurasia, S. P., Singh, K.; George, S. Indian. Chem. Eng. 2010, 51, 300-308.
CrossRef - Jain, S.; Sharma M. P.; Rajvanshi, S. Fuel. Process. Technol. 2011, 92, 32-38.
CrossRef - Bondioli, P. Top. Catal. 2004, 27, 77-82.
CrossRef - Sharma, Y. C.; Singh, B.; Korstad, J. Fuel. 2011, 90, 1309-1324.
CrossRef - Taufiq-yap, Y. H.; Lee, H. V.; Yunus, R.; Juan, J. C. Chem. Eng. J. 2011a, 178, 342-347.
CrossRef - Taufiq-yap, Y. H.; Lee, H. V.; Hussein, M. Z.; Yunus, R. Biomass. Bioenerg. 2011b, 35, 827-834.
CrossRef - Albuquerque, M. C. G.; Urbistondo, I. J.; Gonza´lez, J. S.; Robles, J. M. M., Tost, R. M.; Castello´n, E. R.; Lo´pez, A. J.; Azevedo, D. C. S.; Cavalcante Jr, C. L.; Torres, P. M. Appl. Catal. A Gen. 2008a, 334, 35-43.
CrossRef - Albuquerque, M. C. G.; Gonza´lez, J. S.; Robles, J. M. M.; Tost, R. M.; Castello´n, E.R.; Lo´pez, A. J.; Azevedo, D. C. S.; Cavalcante Jr, C. L.; Torres, P. M. Appl. Catal. A Gen. 2008b, 347, 162-168.
CrossRef - Rubio, A. C. A.; Castillo, M. L. A.; Albuquerque, M. C. G.; Mariscal, R.; Cavalcante Jr, C. L.; Granados, M. L. Fuel. 2012, 95, 464-470.
CrossRef - Pandiangan, K. D.; Jamarun, N.; Arief, S.; Simanjuntak, W. Orient. J. Chem. 2016, 32(2), 385-390.
CrossRef - Boey, P. L., Maniam, G. P.; Hamid, S. A. Chem. Eng. J. 2011, 168, 15-22.
CrossRef - Pandiangan, K. D.; Jamarun, N.; Arief, S.; Simanjuntak, W.; Rilyanti, M. Orient. J. Chem. 2016, 32(2), 3021-3026.
CrossRef - Kouzu, M.; Hidaka. J. Fuel, 2012, 93, 1-12.
CrossRef - Witoon, T.; Bumrungsalee, S.; Vathavanichkul , P.; Palitsakun, S. Bioresour. Technol. 2014, 156, 329-334.
CrossRef - Pandiangan, K. D.; Arief, S.; Jamarun, N.; Simanjuntak, W. J. Mater. Env. Sci. 2017, 8, 1797-1802.
- Chouhan, A. P. S.; Sarma, A. K. Renew. Sust. Energ. Rev. 2011, 15, 4378-4399.
CrossRef - Sivasamy, A.; Cheah, K. Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Chem. Sust. Chem. 2009, 2, 278-300.
CrossRef - Mohadesi, M.; Hojabri Z.; Moradi, G. Biofuel Res. J. 2014, 1, 30-33.
CrossRef - Pandiangan, K. D.; Simanjuntak, W. Indones. J. Chem. 2013, 13, 47-52.
CrossRef - Simanjuntak, W.; Sembiring, S.; Pandiangan, K. D.; Syani, F.; Situmeang, R. T. M. Orient. J. Chem. 2016, 32(3) 2079-2085.
CrossRef - Samart, C.; Chaiya, C.; Reubroycharoen, P. Energy Convers. Manage. 2010, 51, 1428-1431.
CrossRef - Abdulkadir, B. A.; Uemura, Y.; Ramli, A.; Osman, N. Bt.; Kusakabe, K.; Kai. T. Aust. J. Basic. Appl. Sci. 2014, 8, 445-451.
- Omorogbe, S. O.; Ikhuoria, E. U.; Aigbodion, A. I.; Obazee, E. O.; Momodu, V. M. Curr. Res. Chem. 2013, 5, 11 – 18.
CrossRef - Bello, E. I.; Otu. F. Br. J. Appl. Sci. Technol. 2015, 6, 261-275.
CrossRef - Tariq, M.; Ali, S.; Ahmad, F.; Ahmad, M.; Zafar, M.; Khalid, N.; Khan, M. A. Fuel. Process. Technol. 2011, 92, 336-341.
CrossRef - Morshed, M.; Ferdous, K.; Khan, M. R.; Mazumder, M. S. I.; Islam M. A.; Uddin, Md. T. Fuel, 2011, 90, 2981-2986.
CrossRef - Horst, M. T.; Urbin, S.; Burton, R.; McMillan. C. Lipid Tech. 2009, 21, 39-41.

This work is licensed under a Creative Commons Attribution 4.0 International License.









