Green Synthesis and In Vitro Anti Oxidant Activity of 6-aryl-quinazolin-4(3H)-ones via Suzuki Cross Coupling
Shankaraiah Pagilla1 , Siddhartha Marupati1 and Kishore Nani2
, Siddhartha Marupati1 and Kishore Nani2
1Department of Freshman Engineering, Vardhaman College of Engineering, Shamshabad, Kacharam, Hyderabad, Telangana-500018, India.
2Department of Science and Humanities, MLR Institute of Technology, Dundigal, Hyderabad, Telangana-500043, India.
Corresponding Author E-mail: shankarpagilla@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330645
Suzuki cross coupling reaction based synthetic method for the preparation of 6-aryl-quinazolin-4(3H)-ones (3a-h) in ethylene glycol dimethyl ether is described by coupling of 6-iodo-2-phenyl-3-methyl-quinazolin-4(3H)-one (1) with various 6-aryl boranic acids 3 under microwave irradiation These compounds were screened for anti-oxidant activity.
KEYWORDS:6-iodoquinazolin-4(3H)-one, aryl boranic acid, Pd(PPh3)4, 1,1-diphenyl-2-picryl- hydrazil (DPPH), ABTS*+ (2, 2’-azino-bis (3-ethylben - zthiazoline- 6-sulphonic acid), Suzuki coupling, Ethylene glycol dimethyl ether, Microwave irradiation, Anti oxidant activity
Download this article as:| Copy the following to cite this article: Pagilla S, Marupati S, Nani K. Green Synthesis and In Vitro Anti Oxidant Activity of 6-aryl-quinazolin-4(3H)-ones via Suzuki Cross Coupling. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Pagilla S, Marupati S, Nani K. Green Synthesis and In Vitro Anti Oxidant Activity of 6-aryl-quinazolin-4(3H)-ones via Suzuki Cross Coupling. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=39793 |
Introduction
The recent literature is enriched with progressive findings about quinazolin-4(3H)-one and its derivatives which exhibit a broad range of biological properties such as antioxidant1, anti-tumour2, anti-inflammatory3, anti-malarial4, antibacterial5 and anticonvulsant activity6. Further, quinazolin-4(3H)-one have also been investigated scaffold for the synthesis of various drugs and their intermediates7.
Cross coupling reaction is an significant method of generating carbon-carbon bonds in organic compounds, which is catalyzed by various transition metals. In the past three decades, carbon- carbon bond construction reaction has allowed chemists to produce complex molecular structures of various interests including total synthesis of natural products8, medicinal chemistry and industrial process development9.
In modern days, microwave-assisted organic synthesis (MAOS) has fascinated the interest of synthetic chemists. The rate of a reaction is speed up under microwave irradiation compared to conventional heating.
Insight of quinazolin-4(3H)-one moiety and cross coupling reaction, we have in the previous reported the successful use of Kumada cross-coupling reactions for the synthesis of 6-arylquinazolin-4(3H)-ones derivatives10. However, this coupling is related with extended reaction times and with low yields. In search of our previous investigations toward developing more proficient protocol for the synthesis of 6-arylquinazolin-4(3H)-one derivatives, we optimized the Suzuki reaction conditions under microwave irradiation and conventional heating. It was envisaged that the microwave irradiation would enhance the rate of reaction, thereby reducing time.
Herein, we report the coupling of 6-iodo-3-methyl-2-phenyl-quinazolin-4(3H)-one (1) with aryl boranic acids in presence of Pd(PPh3)4 as catalyst derivatives under microwave irradiation as well as conventional heating conditions and evaluated their in vitro antioxidant property.
Experimental Section
IR spectra for all the compounds were recorded in solid KBr on infra cold model 337 Perkin-Elmer instrument. Melting points were measured in open capillary tubes and are uncorrected. The 1H NMR (400 MHz) and 13C NMR (75 MHz) spectra were recorded on Varian Gemini 400 or Bruker 75 in CDCl3 using tetramethylsilane as internal standard. The purity of all obtained compounds was checked by thin-layer chromatography. High Resolution Mass spectra were recorded on Q-TOF mass spectrometer. Multisynth series microwave system (Milestone) were used for Suzuki reaction.
General Procedure for the Preparation of 3a-h via Suzuki Cross Coupling Reaction:
Microwave Heating
The 6- iodoquinazolinone 1 (0.5 mmol) and boronic acid (0.55 mmol) were dissolved ethylene glycol dimethyl ether in a microwave vial. Pd(PPh3)4 (0.02 mmol) and Na2CO3 (1.25 mmol) were added, and the reaction mixture was irradiated in a microwave apparatus at 80 °C, 250 W for 20 min. After the reaction mixture was cooled to ambient temperature, the product was filtered, the filtrate was concentrated, and the crude mixture was purified by silica gel column chromatography using hexane/ethyl acetate (88/12) as eluent.
Conventional Heating
The 6- iodoquinazolinone 1 (0.5 mmol), boronic acid 2 (0.55 mmol) and Na2CO3 (1.25 mmol) were dissolved in ethylene glycol dimethyl ether in a seal tube. After degassing reaction mixture, Pd(PPh3)4 (0.02 mmol) and were added. And the reaction mixture was heated at 80 °C, 250 W for overnight. After the reaction mixture was cooled to ambient temperature, the product was filtered, the filtrate was concentrated, and the crude mixture was purified by silica gel column chromatography using hexane/ethyl acetate (88/12) as eluent.
Assay of anti-oxidant Property
Lipid Peroxidation Assay
In vitro lipid peroxidation of the compounds was determined by the described method11, 12. Briefly, 1 ml of rat liver microsomal fraction was added to 1.0 ml of 150 mM Tris-HCl buffer (pH 7.4) containing various concentrations (50, 100 and 150 µg/ ml) of test compounds, 0.2 ml FeCl3 (1 mM) and 0.2 ml ascorbic acid (0.5 mM) to induce lipid peroxidation. The mixtures were incubated at 37°C for 30 minutes. At the end of the incubation, 0.5 ml of glacial acetic acid and 0.5 ml of 0.33% TBA were added to each mixture. The mixtures were kept in a water bath at 97°C for 45 minutes; at cooling, the pink chromogen was extracted with 2 ml of butanol. The absorbance of the organic layer was measured at 535 nm, and the thiobarbituric acid reactive substances produced were estimated using the MDA standard curve. BHA was used as a control substance (20 µg/ml).
DPPH (1, 1-Diphenyl-2-picrylhydrazyl) Radical Scavenging Activity
The free radical scavenging activity of isolated compounds was measured by 1,1-diphenyl-2-picryl- hydrazil (DPPH) method described by Blois 1958. 0.1 mM solution of DPPH in methanol was prepared and 1ml of this solution was added to 3 ml of various concentrations of (50, 100 and 150 µg/ ml) test compounds and the reference compound (20 µg/ml). After 30 min, absorbance was measured at 517nm. BHA was used as the reference material. All the tests were performed in triplicate. The percentage of inhibition was calculated by comparing the absorbance values of the control and test samples.
% DPPH radical scavenging = [(Absorbance of control – Absorbance of test sample)/ (Absorbance of control)] ×100
ABTS*+ (2, 2’-azino-bis (3-ethylben – zthiazoline- 6-sulphonic acid) radical cation Decolourisation Assay
This method was carried out by well described method13. ABTS*+ (54.8 mg) was dissolved in 50ml of distilled water to 2mM concentration and potassium persulphate (17 mM, 0.3 ml) was added. The reaction mixture was left to stand at room temperature overnight in dark before use. To 0.2 ml of various concentrations (50, 100 and 150 µg/ ml) of test samples and reference standard BHA (20 µg/ml) 1.0 ml of distilled DMSO and 0.16 ml of ABTS*+ solution was added to make a final volume of 1.36 ml. Absorbance was measured spectrophotometrically, after 20 min at 734 nm14.
Spectral Data
3-Methyl-2,6-diphenylquinazolin-4(3H)-one (3a)
1H NMR (400MHz, CDCl3): δ 8.56 (s, 1H), 8.02 (d, 1H, J = 8.6 Hz), 7.82 (d, 1H, J = 8.6 Hz), 7.72 (d, 2H, J = 7.5 Hz), 7.47–7.60 (m, 7H), 7.38 (m, 1H), 3.53 (s, 3H); 13C NMR (75MHz, CDCl3): δ 162.7, 155.9, 146.4, 139.8, 139.6, 135.3, 133.1, 130.0, 128.9, 128.8, 127.9, 127.8, 127.1, 124.5, 120.7, 34.1; IR (KBr): 1680 cm-1; ESI-HRMS: [M+H]+ m/z calculated for C21H17N2O: 313.1263; found at m/z 313.1343. mp 105–106°C.
3-Methyl-2-phenyl-6-(o-tolyl)quinazolin-4(3H)-one (3b)
1H NMR (400MHz, CDCl3): δ 8.31 (s, 1H), 7.73-7.80 (m, 2H), 7.55-7.60 (m, 5H), 7.31 (m, 4H), 3.52 (s, 3H), 2.32 (s, 3H); 13C NMR (75MHz, CDCl3): δ 162.8, 156.1, 146.1, 140.9, 140.5, 135.6, 135.4, 135.3, 130.0, 128.9, 128.0, 127.8, 127.2, 126.8, 125.9, 120.3, 34.3, 20.5; IR (KBr): 1674 cm-1; ESI-HRMS: [M+H]+ m/z calculated for C22H19 N2O: 327.1419; found at m/z 327.1481. mp 136 – 138°C.
6-(4-Methoxyphenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3c)
1H NMR (400MHz, CDCl3 ): δ 8.51 (s, 1H), 7.99 (m, 2H), 7.57-7.65 (m, 7H), 7.02 (d, 2H, J = 7.9Hz), 3.87 (s, 3H), 3.54 (s, 3H); 13C NMR (75MHz, CDCl3): δ 162.7, 159.5, 155.7, 145.8, 139.4 ,135.2 132.8, 132.0, 130.0, 128.8, 128.2, 128.0, 127.8, 123.7, 120.6, 114.3, 55.3, 34.2; IR (KBr): 1674 cm-1. ESI-HRMS: [M+H]+ m/z calculated for C22H18N2O2: 343.13683; found at m/z 343.1448. mp 161 – 162°C.
6-(2-Chlorophenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3d)
1H NMR (400MHz, CDCl3 ): δ 8.40 (s, 1H), 8.05 (d, 1H, J = 7.9 Hz), 7.91 (d, 1H, J = 7.9 Hz), 7.58-7.27 (m, 9H), 3.55 (s, 3H). 13CNMR (75MHz, CDCl3): δ 162.5, 156.4, 146.5, 139.2, 138.3, 135.7, 135.2, 132.5, 131.4, 130.17, 130.08, 129.07, 128.0, 127.34, 127.02, 127.4, 120.3, 34.3. ESI-HRMS: [M+H]+ m/z calculated for C21H16ClN2O: 347.0873; found at m/z 347.0959. mp 110 – 111°C.
6-(2,3-Dichlorophenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3e)
1H NMR (400MHz, CDCl3): δ 8.50 (d, 1H, J = 2.1Hz), 7.95 (dd, 1H, J = 8.5Hz ), 7.82 (d, 1H, J = 8.3 Hz), 7.55-7.60 (m, 7H), 7.39 (m, 1H), 3.54 (s, 3H); 13C NMR (75MHz, CDCl3): δ 162.5, 156.6, 147.2, 136.9, 135.6, 135.5, 135.1, 132.8, 130.2, 128.9, 128.4, 127.9, 127.6, 125.6, 124.8, 34.4; IR (KBr): 1674 cm-1. ESI-HRMS: [M+H]+ m/z calculated for C21H15 Cl2N2O2: 381.0483; found at m/z 381.0549. mp 222-224°C.
6-(4-Fluorophenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3f)
1H NMR (400MHz, CDCl3) δ 8.49 (s, 1H), 7.95 (d, 1H, J = 8.4Hz), 7.81 (d, 1H, J = 8.3Hz), 7.55-7.69 (m, 7H), 7.15-7.199 (m, 2H), 3.51 (s, 3H); 13C NMR (75MHz, CDCl3): δ 162.6, 156.4, 146.4, 138.7, 135.7, 135.2, 132.9, 130.0, 128.8, 127.9, 124.2, 120.6, 115.9, 115.6, 34.2; IR (KBr): 1680 cm-1. ESI-HRMS: [M+H]+ m/z calculated for C21H16ClN2O: 347.0951; found at m/z 347.0959. mp 195 – 196°C.
6-(3-Fluoro-4-methoxyphenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3g)
1HNMR (400MHz, CDCl3) δ 8.49 s (s, 1H), 7.95 (m, 2H) 7.44-7.69 (m, 7H), 7.07 (m, 1H), 3.96 (s, 3H), 3.55 (s, 3H); 13CNMR (75MHz, CDCl3): δ 162.7, 155.9, 154.2, 150.9, 146.3, 138.2, 135.2, 132.6, 130.0, 128.8, 127.9, 123.9, 122.8, 120.6, 114.9, 114.6, 113.6, 56.3, 34.3; IR (KBr): 1679 cm-1; ESI-HRMS: [M+H]+ m/z calculated for C21H15FN2O: 331.1168; found at m/z 331.1242. mp 159 – 160°C.
3-Methyl-6-(naphthalen-1-yl)-2-phenylquinazolin-4(3H)-one (3h)
1H NMR (400MHz, CDCl3) δ 8.49 (d, 1H, J = 1.7 Hz), 7.86-7.95 (m, 5H), 7.43-7.63 (m, 9H), 3.54 (s, 3H); 13CNMR (75MHz, CDCl3) δ162.7, 146.7, 146.5, 139.7, 138.7, 136.3, 133.8, 131.4, 130.1, 128.9, 128.4, 128.2, 128.0, 127.6, 127.4, 126.4, 125.5, 125.4, 120.5, 34.3; IR (KBr): 1674 cm-1.;ESI-HRMS: [M+H]+ m/z calculated for C25H18N2O: 363.1419; found at m/z 363.1485. mp 111-113°C.
Result and discussion
6-Iodo-3-methyl-2-phenyl-quinazolin-4(3H)-one (1) was chosen as the scaffold which inturn was prepared in previous reported procedure. The compound 1 was characterized by 1H NMR and HRMS spectral techniques10.
 |
Scheme 1 Click here to View scheme |
Microwave-assisted Suzuki Cross-coupling reaction of 6-iodo-quinazolin-4-(3H)-one (1) is explored for the first time with a set of eight aryl boranic acid 2 to provide 6-aryl-quinazolin-4-(3H)-ones 3a. Primarily, the evaluation of solvent was studied using 6-iodo-quinazolin-4(3H)-one 1 (1.0 equiv) boranic acid 6a (1.1 equiv) and Na2CO3 7 (1.1equiv). Employing solvents (Entry 1, 2, 3, 4, 5, and 6) resulted in the required compound 3a with inferior yields. The reaction of 1 with aryl boranic acids 2a in presence of 10 mol% of Pd(PPh3)4 as catalyst for 20 min in Ethylene glycol dimethyl ether gave 3a with 95% yield (Scheme-1). The same product 6a was also obtained (90%) under conventional heating (80 °C) in toluene for 10 h (Table 1, entry 1).
Table 1: Optimized conditions for 3a compound with different solvents.
| Entry | Solvent | Yield | |
| 80°C | MW | ||
| 1. | DMF | 20 | 40 |
| 2. | DMF:Water (4:1) | 40 | 60 |
| 3. | Toulene | 37 | 40 |
| 4. | Toluene:Water | 60 | 66 |
| 5. | 1,4-Dioxane | 33 | 43 |
| 6. | 1,4-Dioxane:Water | 62 | 71 |
| 7. | Ethylene glycol dimethyl ether | 85 | 95 |
The spectral identification of 3a has been discussed below. The IR (KBr) spectrum of 3a showed a strong absorption band at 1680 cm-1 corresponding to carbonyl group. The 1H NMR spectrum (CDCl3, 400 MHz) of 3a showed a singlet at δ 8.56 for C-5 proton, a doublet at δ 8.02 corresponds to C-7 proton. A singlet peak at δ 3.53 integrating for three protons is due to N-CH3 group. The peaks for the remaining eleven aromatic protons observed at δ 7.82 (d, J = 8.6 Hz, 1H), δ 7.72 (d, J = 7.50 Hz, 2H), δ 7.47-7.60 (m, 7H) and in the range δ 7.38-7.41(m, 1H). 13C-NMR spectrum (CDCl3, 100 MHz) of 3a showed signals at δ 162.7 due to carbonyl carbon, δ 155.9 for C-2 carbon, and a peak at δ 34.3 due to N-methyl carbon. Further 13C NMR spectrum showed signals at δ 146.4, 139.8, 139.6, 135.3, 133.1, 130.0, 128.9, 128.8, 127.9, 127.8, 127.1, 124.5, 120.7 due to the remaining aromatic carbons. The structure was further confirmed by high resolution Mass spectrum of 3a indicates [M+H]+ peak at m/z 313.1349 due to the molecular formulae C21H17N2O.
This Suzuki coupling reaction of 1 was extended to remaining substituted phenyl boranic acids 2b-h and the corresponding 6-arylquinazolin-4(3H)-ones (3b-h) were isolated in 93-95% yield (Scheme-1, Table-2). These compounds 3b-h were characterized based on their IR, 1H NMR, 13C NMR and HRMS spectral data
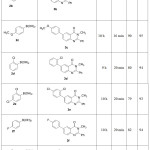 |
Table 2: Synthesis of 6-substituted quinazolinone using Suzuki cross coupling Click here to View table |
Assay of Antioxidant Property
Lipid Peroxidation Assay
6-(2,3-Dichlorophenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3e) and 6-(3-Fluoro-4-methoxyphenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3g) exhibited concentration-dependent FeSO4 induced lipid peroxidation and showed highest percentage of inhibition with that from other compounds and comparable to reference drug BHA. P < 0.5 (Table 4).
DPPH Radical Scavenging Activity
Among the tested compounds 6-(2,3-Dichlorophenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3e) and 6-(3-Fluoro-4-methoxyphenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3g) are showed highest percentage of inhibition which was comparable with standard BHA. 3-Methyl-2,6-diphenylquinazolin-4(3H)-one (3a), 3-Methyl-2-phenyl-6-(o-tolyl)quinazolin-4(3H)-one (3b), 6-(4-Methoxyphenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3c), 6-(2-Chlorophenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3d), 6-(4-Fluorophenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3f) and 3-Methyl-6-(naphthalen-1-yl)-2-phenylquinazolin-4(3H)-one (3h) are also possessed considerable anti-oxidant properties P<0.5 (Table-3 ).
ABTS*+ radical Cation Decolourisation Assay
Synthesized compounds showed good scavenging of ABTS*+ radical at all tested concentrations (Table-3). The highest inhibition was achieved with 6-(4-Fluorophenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3f) comparing to other compounds P<0.5. Whereas, 6-(3-Fluoro-4-methoxyphenyl)-3-methyl-2-phenylquinazolin-4(3H)-one (3g) was the next compound showed significant at 150 µg/ml (Table-3).
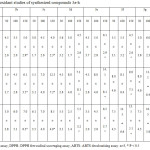 |
Table 3: In vitro anti-oxidant studies of synthesized compounds 3a-h Click here to View table |
 
L.P.O –Lipid Peroxidation assay, DPPH- DPPH free radical scavenging assay, ABTS- ABTS decolourising assay. n=3, * P < 0.5
Conclusion
A more efficient and novel C-C bond forming, Pd catalyzed Suzuki coupling reaction is developed as a new method for the preparation 6-aryl-quinazolin-4(3H)-ones (3a-h) under microwave irradiation conditions in ethylene glycol dimethyl ether has been achieved with good yields over conventional method. According to data obtained from the present study, these quinazolin-4(3H)-one derivatives could be considered as useful templates for further development to obtain In vitro antioxidant activity
Acknowledgments
Shankaraiah P and Siddhartha Marupati thank Vardhaman College, Shamshabad, Kacharam, Hyderabad, Telangana for providing financial assistance.
References
- Priya, M. G. R.; Girija, K.; Ravichandran, N. Rasayan Journal of Chemistry 2011, 4, 418-424.
- Noolvi, M. N.; Patel, H. M. Arabian Journal of Chemistry 2013, 6, 35-48.
CrossRef - Salgın-Gökşen, U.; Gökhan-Kelekçi, N.; Göktaş, Ö.; Köysal, Y.; Kılıç, E.; Işık, Ş.; Aktay, G.; Özalp, M. Bioorganic & medicinal chemistry 2007, 15, 5738-5751.
CrossRef - Birhan, Y. S.; Bekhit, A. A.; Hymete, A. BMC research notes 2015, 8, 589.
CrossRef - Fathalla, O. A. E.-F. M.; Kassem, E. M.; Ibrahem, N. M.; Kamel, M. M. Acta Pol Pharm 2008, 65, 11-20.
- Paneersalvam, P.; Raj, T.; Ishar, M.; Singh, B.; Sharma, V.; Rather, B. Indian journal of pharmaceutical sciences 2010, 72, 375.
CrossRef - Abida, P. N.; Arpanarana, M. International Journal of Pharmaceutical & Biological Archive 2011, 2, 1651-1657.
- Chemler, S. R.; Trauner, D.; Danishefsky, S. J. Angewandte Chemie International Edition 2001, 40, 4544-4568.
CrossRef - Johansson Seechurn, C. C.; Kitching, M. O.; Colacot, T. J.; Snieckus, V. Angewandte Chemie International Edition 2012, 51, 5062-5085.
CrossRef - Shankaraiah, P.; Veeresham, S.; Bhavani, A. Russian Journal of General Chemistry 2016, 86, 368- 375.
CrossRef - Newman, D. J.; Cragg, G. M.; Snader, K. M. Natural product reports 2000, 17, 215-234.
CrossRef - Butler, M. S. Journal of natural products 2004, 67, 2141-2153.
CrossRef - Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Free radical biology and medicine 1999, 26, 1231-1237.
CrossRef - Magaldi, S.; Mata-Essayag, S.; De Capriles, C. H.; Perez, C.; Colella, M.; Olaizola, C.; Ontiveros, Y. International Journal of Infectious Diseases 2004, 8, 39-45.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









