Antitermitic Activity of Cinnamomum Parthenoxylon leaves Against Coptotermes Curvignathus
Morina Adfa1 , Anwar Sanusi1, Syalfinaf Manaf2, Irfan Gustian1 and Charles Banon1
, Anwar Sanusi1, Syalfinaf Manaf2, Irfan Gustian1 and Charles Banon1
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, University of Bengkulu, Jalan W.R. Supratman, Kandang Limun, Bengkulu 38371, Indonesia.
2Department of Biology, Faculty of Mathematics and Natural Sciences, University of Bengkulu, Jalan W.R. Supratman, Kandang Limun, Bengkulu 38371, Indonesia.
Corresponding Autthor E-mail: morinaadfa@unib.ac.id
DOI : http://dx.doi.org/10.13005/ojc/330646
The antitermitic activity of Cinnamomum parthenoxylon leaves crude methanol extract and its fractions against Coptotermes curvignathus was investigated in a laboratory test. A no-choice test was employed for determining antitermitic activity. The results showed that all fractions and crude methanol extract at concentrations tested was reduced the survival of termite and as well as showed feeding deterrent activity compares to the corresponding control. The results of the preliminary phytochemical screening, and refer to previously reported data the secondary metabolites present in the crude MeOH extract and EtOAc fractions are terpenoids, steroids, flavonoids, coumarin, phenolics, benzenoids, chromene, and fatty acid methyl esters. Base on GC-MS data, the chemical components of n-hexane fraction showed 13 components which was divided into five groups, fatty acid methyl esters (53.66%), 1,2-benzenedicarboxylic acid, bis (2-ethylhexyl) ester (31.17%), phytol (6.49%), sesquiterpenes (6.47%), and a phenyl propanoid (2.22%). Need further investigation to examine which compounds are responsible for termiticidal and antifeedant activity, either singly or jointly.
KEYWORDS:Cinnamomum Parthenoxylon; Antitermitic; Termiticidal; Antifeedant; GC-MS and Coptotermes Curvignathus
Download this article as:| Copy the following to cite this article: Adfa M, Sanusi A, Manaf S, Gustian I, Banon C. Antitermitic Activity of Cinnamomum parthenoxylon leaves Against Coptotermes Curvignathus. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Adfa M, Sanusi A, Manaf S, Gustian I, Banon C. Antitermitic Activity of Cinnamomum parthenoxylon leaves Against Coptotermes Curvignathus. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40874 |
Introduction
Cinnamomum is one of the large genera of Lauraceae, with more than 250 species worldwide, and is distributed in the tropical and subtropical region of Asia, China, Australia, and America1. The antioxidant, antimicrobial, anticancer, and insecticidal activities of some cinnamomum species has been extensively investigated2-5. Cinnamomum cassia is the famous one, the main constituents of C. cassia barks oil are cinnamaldehyde and used as an antibacterial agent, antifungal, antidiabetic, and to be insecticidal to adults of Tribolium castaneum and Sitophilus zeamais, and to T. castaneum larvae2.
Cinnamomum parthenoxylon is widely distributed in South-Eastern Asia. The wood is used for general construction and furniture materials. It is resistant to insect attack because of its persistent smell. The leaves, stem barks, roots, and stem woods of C. parthenoxylon have shown significant antibacterial activity against a wide range of Gram positive and Gram negative bacteria, antioxidant, antifungal, and anticancer activities6-9.
Only a few reported on the effects of Cinnamomum spp. on termite control. C. camphora exhibited effective repellent performance and feeding deterrent toward Coptotermes curvignathus10, whereas C. camphora oil was shown low toxicity against dry wood termite (Cryptotermes brevis)11. The essential oils of C. osmophloeum and C. zeylanicum leaves exhibited great termite resistance12, and cinnamaldehyde, eugenol, and terpineol in C. osmophloeum leaf essential oil exhibited high antitermite activity against C. formosanus13.
Thus far, to the best of our knowledge, there are no reports describing the antitermite activity of C. parthenoxylon. In continuation of our study for natural pesticides in plants14-16, in this study, the antitermite activity of C. parthenoxylon leaves crude methanol extract and its fractions against C. curvignathus was investigated, and their chemical components were analyzed.
Materials and Methods
General
GC/MS data were measured with a SHIMADZU QP-2010 GCMS, the condition of GC-MS according to previously method15: a 30 m capillary column DB 1 Agilent (J and W, USA) with id 0.25 mm and 0.1 μm film thickness was used; column temperature from 70°C (5 min) to 260°C (17 min) at 5°C/min; injection temperature of 310°C, and helium as the carrier gas. The percentage of components was calculated by the GC peak area. The mass spectrometer was operated in EI mode at 70 eV. The mass spectra were obtained by ACQ mode scan of the mass range from 20 to 600. The injection temperature and ionization source temperature were 305 and 250°C, respectively. The compounds were identified based on the comparison of their retention time (RT) and mass spectra of Wiley, NIST library data of the GC-MS system.
Plant Material and Termites
The leaves of C. parthenoxylon were collected around University of Bengkulu, Bengkulu city, Indonesia. The plant was determined at the Herbarium Bogoriense, Indonesian Institute of Science (LIPI) Bogor, Indonesia. A voucher specimen (MAKG-01) has been deposited at the Laboratory of Organic, Department of Chemistry, University of Bengkulu. The termites for a test are Coptotermes curvignathus Holmgren were obtained from the plants that attacked by termites around University of Bengkulu, and termites were fed wet filter papers before the test.
Extraction, Fractionation, and Phytochemical Screening
Fresh leaves of C. parthenoxylon (1.4 Kg) were chopped and macerated at room temperature in methanol (5×5.5 L), then the mixture was filtered and concentrated in a rotary evaporator to yield a crude methanol extract (84.5 g). The crude MeOH extract (42.3 g) was dissolved in methanol and distilled water (1:1), and fractionated with n-hexane, and ethyl acetate, successively. Removal the solvents in vacuo afforded the n-hexane (10 g), ethyl acetate (EtOAc) (6.2 g), and methanol-water (MeOH-H2O) fractions (21.4 g). Phytochemical screening were performed using standard procedures17.
Antitermite Test
A no-choice test was employed for evaluating antitermite activity according to previously described method15. The concentration of crude MeOH extract, n-hexane, EtOAc, and MeOH-H2O fractions of C. parthenoxylon was prepared to 5%, 10%, and 15% (sample mass/filter paper mass×100%). The samples were dissolved in 300 µL n-hexane or methanol, the resulting solution was applied to filter papers (3×3 cm, Whatman No. 1). 20 workers and 2 soldiers were used for tested. The number of dead termites was counted daily for 2 weeks, and the mass loss of filter paper was calculated in the end of tested. Three replications were performed for each sample. The termicidal activity was evaluated from the mortality (%) average, and the antifeedant activity were evaluated from mass loss (%) of filter paper.
Results and Discussion
C. parthenoxylon leaves methanol extract was separated into three fractions of increasing polarity by fractionation using n-hexane, and ethyl acetate. We evaluated the antitermitic activity crude MeOH extract of C. parthenoxylon leaves and its fractions against C. curvignathus, the results were shown in Table 1. No choice forced feeding of C. curvignathus on a filter paper impregnated with 5%, 10%, and 15% of the crude MeOH extract, n-hexane, EtOAc, and MeOH-H2O fractions resulted in increased termite mortality with increasing extract and fractions concentrations. All termite dead within 2 weeks for crude MeOH extract, n-hexane and EtOAc fractions at a concentration of 15%, while MeOH-H2O fraction (15%) showed 87.88% mortality after 2 weeks. All fractions and crude methanol extract reduced the survival of termite compares to the control.
Filter paper consumption by the termites after 2 weeks examine to crude MeOH extract of C. parthenoxylon leaves and its fractions were compared with that control (Table 1). All fractions and crude methanol extract showed feeding deterrent activity. Average mass loss of filter paper treated with crude MeOH extract and fractions at all concentrations tested was lower than the control, average mass loss of filter paper was decreased as the concentration increased. A similar termiticidal and antifeedant effects were obtained between n-hexane and EtOAc fractions, respectively.
Table 1: Antitermite activity of C. parthenoxylon leaves crude MeOH extract and its fractions against Coptotermes curvignathus
| Crude MeOH extract and its fractions | Mortality of termite (%) | Mass loss (%)of | |
| recorded over time | filter paper | ||
| 7 days | 14 days | 14 days | |
| Control (MeOH) | 3.03 | 6.06 | 19.07 |
| Control (n-Hexane) | 1.52 | 4.55 | 18.53 |
| Crude MeOH extract 5% | 37.88 | 87.88 | 14.4 |
| Crude MeOH extract 10% | 42.42 | 89.39 | 12.4 |
| Crude MeOH extract 15% | 65.15 | 100 | 8.3 |
| n-Hexane fraction 5% | 42.42 | 78.79 | 12.0 |
| n-Hexane fraction 10% | 63.64 | 95.45 | 10.3 |
| n-Hexane fraction 15% | 81.82 | 100 | 8.5 |
| EtOAc fraction 5% | 53.03 | 72.73 | 12.4 |
| EtOAc fraction 10% | 68.18 | 90.91 | 11 |
| EtOAc fraction 15% | 83.33 | 100 | 8.7 |
| MeOH-H2O fraction 5% | 15.15 | 53.03 | 14.1 |
| MeOH-H2O fraction 10% | 31.82 | 71.21 | 12.5 |
| MeOH-H2O fraction 15% | 48.48 | 87.88 | 10.5 |
The crude methanol extract of C. parthenoxylon leaves was subjected to preliminary phytochemical screening (Table 2). The presence of secondary metabolites such as flavonoids, phenolics, terpenoids/steroids, and coumarins on C. parthenoxylon leaves might be responsible for antitermite activity, because some classes of flavonoids and terpenoids have been reported as termiticidal and feeding deterrent to termite18-19.
The analysis of chemical components of the n-hexane fraction of C. parthenoxylon leaves was carried out using GC-MS. The n-hexane fraction was contain 13 components, and the components are listed in Table 3. The n-hexane fraction was characterized by a high content of fatty acid methyl esters 53.66% (hexadecanoic acid, methyl ester; 10-octadecenoic acid, methyl ester; 9,12,15-octadecatrienoic acid, methyl ester (Z,Z,Z); 9,12-octadecadienoic acid, methyl ester (E,E); 9-octadecenoic acid,12-hydroxy, methyl ester (9Z,12R); octadecanoic acid, methyl ester), followed by 1,2-benzenedicarboxylic acid, bis (2-ethylhexyl) ester (31.17%), phytol (6.49%), sesquiterpenes 6.47% (β-caryophyllene, δ-guaiene, caryophyllene oxide, patchouli alcohol), and a phenyl propanoid 2.22% (β-asarone).
Several plants essential oils and its components have been reported to show larvicidal, antitermite or repellent activity. β-Caryophyllene and caryophyllene oxide from Plectranthus barbatus and Zanthoxylum avicennae essential oils have larvicidal activity against malaria mosquito vectors20, and Aedes albopictus Skuse21. Zhu et al. (2003) reported that patchouli oil was obtained from Pogostemon cablin and its major component patchouli alcohol have toxic and repellent activity against Coptotermes formosanus 22. The possibility that the toxicity of patchouli oil and patchouli alcohol against termites have a neurotoxic mode action. This result obtained also agree well with Adfa et al. (2015)15 that b-asarone at low concentration in A. calamus rhizome fractions revealed strong termiticidal activity against C. curvignathus. There is a report the presence of 1,2-benzenedicarboxylic acid, bis (2-ethylhexyl) ester in Sterculia guttata seeds and its larvicidal activity against Aedes aegypti and Culex quinquefasciatus larvae23. Although 1,2-benzenedicarboxylic acid, bis (2-ethylhexyl) ester is the member of the class of phthalates which are used as plasticizers medical devices.
Other studies have confirmed that coumarin (scopoletin), flavonoids (isorhoifolin, rutin, nicotiflorin, epicatechin, kaempferol-3-O-rhamnoside, herbacetin, quercetin-3-O-rhamnoside), bezenoids (4-hydroxybenzoic acid, 4-hydroxybenzaldehyde, 1,2,4-trihydroxybenzene), fatty acid methyl esters (hexadecanoic acid methyl ester, 12-hexadecenoic acid methyl ester), sesquiterpene (blumenol A), chromene dimer (3R,4R,3′R,4′R)-6,6′-dimethoxy-3,4,3′,4′-tetrahydro-2H,2’H-[3,3′]bichromenyl-4,4′-diol), and steroid (daucosterol) have been isolated and purified from ethyl acetate fraction of C. parthenoxylon leaves24-25. Steroid (β-sitosterol) was isolated from petroleum ether fraction25. Scopoletin and 4-hydroxybenzaldehyde have been showed good termiticidal activity, and quercetin-3-O-rhamnoside demonstrated moderate antifeedant activity against Coptotermes formosanus13,14,26, whereas 4-hydroxybenzoic acid was claimed as a base material of attractant for termite control agent27.
Table 2: Phytochemical screening of crude MeOH extract of C. parthenoxylon leaves
| Classes of compounds | Test Applied |
Result |
| Alkaloids | Dragendorff | – |
| Flavonoids | Shinoda (Mg/HCl) | + |
| Phenolics | Ferric chloride | + |
| Terpenoids/ and Steroids | Liebermann-Burchard (H2SO4-Ac2O) | + |
| Coumarins | Spot test (NaOH) | + |
Note: + (detected) and – (not detected)
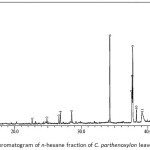 |
Figure 1: GC chromatogram of n-hexane fraction of C. parthenoxylon leaves Click here to View figure |
Table 3: Identified chemical components of n-hexane fraction of C. parthenoxylon leaves
| RT (min) | Compounds | Peak area (%) |
| 22.619 | β-Caryophyllene | 0.95 |
| 24.826 | δ-Guaiene | 0.98 |
| 26.622 | Caryophyllene oxide | 1.53 |
| 26.861 | β-Asarone | 2.22 |
| 28.537 | Patchouli alcohol | 3.01 |
| 34.279 | Hexadecanoic acid, methyl ester | 16.55 |
| 37.542 | 9,12-Octadecadienoic acid, methyl ester (E,E) | 6.21 |
| 37.622 | 9,12,15-Octadecatrienoic acid, methyl ester (Z,Z,Z) | 9.38 |
| 37.717 | 10-Octadecenoic acid, methyl ester | 14.67 |
| 38.255 | Octadecanoic acid, methyl ester | 2.49 |
| 39.116 | Phytol/ 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 6.49 |
| 41.709 | 9-Octadecenoic acid,12-hydroxy, methyl ester (9Z,12R) | 4.36 |
| 45.500 | 1,2-Benzenedicarboxylic acid, bis (2-ethylhexyl) ester | 31.17 |
Conclusion
In conclusion, crude methanol extract of C. parthenoxylon leaves and its fractions showed good termiticidal and antifeedant activity against C. curvignathus, while n-hexane and EtOAc fractions showed nearly similar activity. These results exhibited that the antitermitic activity of the crude methanol extract of C. parthenoxylon leaves and all fractions can be attributed to secondary metabolites present in the extract and fractions. The compounds responsible for antitermitic activity need further investigation, either jointly or independently. C. parthenoxylon leaves crude MeOH extract and its fractions might be potential to develop new bio pesticides and wood preservative agent.
Acknowledgements
Thank to Ministry of Research, Technology and Directorate General of Higher Education, The Republic of Indonesia for a research grant (Contract number 044/SP2H/LT/DRPM/II/2016).
References
- Jayaprakasha, G. K.; Rao, L. J. M. Crit. Rev. Food Sci. Nutr. 2011, 51, 547-562.
CrossRef - Huang, Y.; Ho, S. H. J. Stored Prod. Res. 1998, 34, 11-17.
CrossRef - Jayaprakasha, G. K.; Ohnishi-Kameyama, M.; Ono, H.; Yoshida, M.; Jaganmohan Rao, L. J. Agric. Food Chem. 2006, 4, 1672-1679.
CrossRef - Jantan, I. B.; Karim Moharam, B. A.; Santhanam, J.; Jamal, J. A. Pharm. Biol. 2008, 46, 406-412.
CrossRef - Wong, H. Y.; Tsai, K. D.; Liu, Y. H.; Yang, S. M.; Chen, T. W.; Cherng, J.; Chou, K. S.; Chang, C. M.; Yao, B. T.; Cherng, J. M. Phytother. Res. 2016, 30, 331-340.
CrossRef - Adfa, M.; Rahmad, R.; Ninomiya, M.; Yudha, S. S; Tanaka, K.; Koketsu, M. Bioorg. Med. Chem. Lett. 2016, 26, 761-764.
CrossRef - Buru, A. S.; Pichika, M. R.; Neela, V.; Mohandas, K. J. Ethnopharm. 2014, 153, 587-595.
CrossRef - Phongpaichit, S.; Kummee, S.; Nilrat, L.; Itarat, A. Songklanakarin J. Sci. Technol. 2007, 29, 11-16.
- Kawamura, F.; Ramle, S. F. M.; Sulaiman, O.; Hashim, R.; Ohara, S. Eur. J. Wood Wood Prod. 2011, 69, 207-212.
CrossRef - Roszaini, K.; Nor Azah, M. A.; Mailina, J.; Zaini, S.; Faridz, Z. M. Wood Sci. Technol. 2013, 47, 1273-1284.
CrossRef - Sbeghen, A. C.; Dalfovo, V.; Serafini, L. A.; De Barros, N. M. Sociobiology 2002, 40, 585-593.
- Lin, T. S.; Yin, H. W. Bull. Taiwan Forest. Res. Inst. New Series 1995, 10, 459-464.
- Chang, S. T.; Cheng, S. S. J. Agric. Food Chem. 2002, 50, 1389-1392.
CrossRef - Adfa, M.; Hattori, Y.; Ninomiya, M.; Funahashi, Y.; Yoshimura, T.; Koketsu, M. Nat. Prod. Res. 2013, 27, 270-273.
CrossRef - Adfa, M.; Livandri, F.; Meita, N. P.; Manaf, S.; Ninomiya, M.; Gustian, I.; Putranto, A. M. H.; Supriati, R.; Koketsu, M. J. Asia-Pac. Entomol. 2015, 18, 47-50.
CrossRef - Adfa, M.; Kusnanda, A. J.; Livandri, F.; Rahmad, R.; Darwis, W.; Efdi, M.; Ninomiya, M.; Koketsu, M. Rasayan J. Chem. 2017, 10, 153-159.
- Farnsworth, N. R. J. Pharma. Sci. 1966, 55, 225-276.
CrossRef - Kusumoto, N.; Ashitani, T.; Hayasaka, Y.; Murayama, T.; Ogiyama, K.; Takahashi, K. J. Chem. Ecol. 2009, 35, 635-642.
CrossRef - Ishida, M.; Serit, M.; Nakata, K.; Juneja, L. R.; Kim, M.; Takahashi, S. Biosci. Biotechnol. Biochem. 1992, 56, 1835-1838.
CrossRef - Govindarajan, M.; Rajeswary, M.; Hoti, S. L.; Bhattacharyya, A.; Benelli, G. Parasitol. Res. 2016, 115, 807-815.
CrossRef - Liu, X. C.; Liu, Q. Y.; Zhou, L.; Liu, Q. R.; Liu, Z. L. Trop. J. Pharm. Res. 2014, 13, 399-404.
CrossRef - Zhu, B. C. R.; Henderson, G.; Yu, Y.; Laine, R. A. J. Agric. Food Chem. 2003, 51, 4585-4588.
CrossRef - Katade, S. R.; Pawar, P. V.; Tungikar, V. B.; Tambe, A. S.; Kalal, K. M.; Wakharkar, R. D.; Deshpande, N. R. Chem. Biodiver. 2006, 3, 49-53.
CrossRef - Pardede, A.; Adfa, M.; Kusnanda, A. J.; Ninomiya, M.; Koketsu, M. Med. Chem. Res. 2017, 26, 2074-2079.
CrossRef - Wei, X.; Li, G. H.; Wang, X. L.; He, J. X.; Wang, X. N.; Ren, D. M.; Lou, H. X.; Shen, T. Biochem. Syste. Ecol. 2017, 70, 95-98.
CrossRef - Adfa, M.; Yoshimura, T.; Komura, K.; Koketsu, M. J. Chem. Ecol. 2010, 36, 720-726.
CrossRef - Peterson, J. E. 1998. Patent US5756114 A.

This work is licensed under a Creative Commons Attribution 4.0 International License.









