Synthesis of Some new Nucleosides Derived from 2- Mercapto Benzimidazole With Expected Biological Activity
Hamada. H. Amer,1,2, Omar. M. Ali1,3, and Ibrahim Kh. El-kafaween1
1Department of Chemistry, Faculty of Applied and Medical Sciences, Taif University, Turbah, Taif, Saudia Arabia.
2Animal Medicine and Infectious Diseases Department, Faculty of Veterinary medicine, Sadat City University, Egypt.
3Department of Chemistry, Faculty of Science, Menoufia University, Shebin El-Koam, Egypt.
Corresponding Author E-mail: dr.hamada1435@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330519
2-mercaptobenzimidazole derivatives and their acyclic nucleosides were synthesized. The synthesized compounds were tested for their antibacterial activity against Escherichia coli, Staphylococcus aureus and S. epidermidis. Most of tested compounds showed moderate to high antibacterial activity while few compounds were found to show little or no activity against the tested microorganisms.
KEYWORDS:Oxadiazolines; Arylidene Derivatives; Nucleosides; Antibacterial Activity
Download this article as:| Copy the following to cite this article: Amer H. H, Ali O. M, El-kafaween I. K. Synthesis of Some new Nucleosides Derived from 2- Mercapto Benzimidazole With Expected Biological Activity. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Amer H. H, Ali O. M, El-kafaween I. K. Synthesis of Some new Nucleosides Derived from 2- Mercapto Benzimidazole With Expected Biological Activity. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=39063 |
Introduction
The chemistry of heterocyclic compounds was used to prepare 2-mercaptobenzimidazole and its derivatives for obtaining novel biologically active ingredients such as anthelmintic [1], anti-HIV [2], antifungal [3] antibacterial [4], CNS depressant [5], anti-inflammatory [6], and analgesic [7] activities. In the last years, 2-mercaptobenzimidazole derivatives play an important role in therapeutic and pharmacological fields. It possesses antimicrobial [8], analgesic and anti-inflammatory activities [9]. A number of 2-mercaptobezoimidazole derivatives have been prepared by the general method described by Van Allan and Deacon [10]. The synthized 2-mercaptobenzoimidazole derivatives exhibit moderate antibacterial and antifungal activities when compared to ampicillin and ketoconazole respectively [11]. 2-mercaptobenzimidazole derivatives were screened for their anti-inflammatory activity by carrageenan induced paw edema method [12]. The derivatives of 2-mercaptobenzimidazole were prepared using Mannich reaction showed moderate to high antibacterial activity [13].The benzimidazole moiety is present in wide antimicrobial, antiprotozoal and analgesic drugs [14-16]. The pharmacological and biological evaluating of it is clearly concluded that the prepared compounds are promisingly high antimicrobial, anti-inflammatory and analgesic agent [17]. Benzimidazole derivatives play an important role in medical field with so many Pharmacological activities such as antimicrobial, antiviral, antidiabetic and anticancer activity [18]. Benzimidazole derivatives are considered as a promising class of biologically active heterocyclic compounds that show a wide of biological activities like anti-viral, anti-microbial, anticancer, anti-diabetic, anti-parasitic, anti-oxidant, anthelmintics, anti-HIV, anti-proliferative, anti-convulsant, anti-inflammatory, anti-hypertensive, anti-neoplastic, and anti-trichinellosis [19]. Synthesized Schiff base (AMPOHA) showed hight activity against Bacillus subtilis, Escherichia coli, Aspergillu and Aspergillus flavus [20] . N-phthalimido and acetomido derivatives has to undergo antimicrobial activity against Klebsiella, Escherichia coli, epidermitis, Staphylococcus, Micrococcus leteus, Bacillus cereus and Staphylococcus aureus [21].
Results and Discussion
2-mercaptobenzimidazole (2) was synthized by mannich reaction and allowed to react with ethylchloroacetate in acetone and dry potassium carbonate to afford ethoxycarbonylmethyl-2-mercaptobenzimidazole (3) in 87% yield. Hydrazinolysis of the ethyl ester 3 in etnanol at reflux temperature affording the corresponding hydrazide 4 in 92% yield which was allowed to react with different sugars in ethanol at reflux temperature and in the presence of acetic acid to afford sugar hydrazone derivatives 5a-d in 83-88% yields. Acetylation of 5a-d with acetic anhydride in pyridine at room temperature afforded acetylated sugar hydrazone derivatives 6a-d in 75-92% yields. When the acetylation was carried out in acetic anhydride under reflux at 90 °C afforded oxadiazoline derivatives 7a-d in 70-78% yields. Treatment of hydrazide 4 with different aldehydes to give arylidene derivatives 8a-d in 88-94 % yields. The arylidene derivatives were reacted with acetic anhydride to afford oxadiazoline derivatives 9a-d in 80-88 % yields.
Experimental
Melting points were determined with a Kofler block apparatus and are uncorrected. The IR spectra were recorded on a Perkin-Elmer model 1720 FTIR spectrometer for KBr discs. NMR spectra were recorded on a Varian Gemini 200 NMR Spectrometer at 300 MHz for 1H NMR with TMS as a standard. The progress of the reactions was monitored by TLC using aluminum silica gel plates 60 F 245. Spectroscopic analyses were performed at the Dr.Ahmed Farag laboratory at Faculty of science, Cairo University, Egypt.
Ethoxycarbonylmethyl-2-Mercaptobenzimidazole (3)[22].
A mixture of 2-mercaptobenzimidazole 2 (15g, 0.1 mole), dry acetone (300 ml), anhydrous K2CO3 (13.8 g, 0.1 mole) and ethylchloroacetate (15.92 g, 0.13 mole) was heated under reflux for 6h (TLC). The reaction mixture was filtered of and the filtrate was evaporated under reduces pressure. The residue was recrystallized from ethanol to yield brown oil in 87% yield. Rf = 0.48 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ = 1.26 (t, 3H, J = 8.1 Hz, CH3CH2), 4.15 (q, 2H, J = 8.1 Hz, CH3CH2), 4.55 (s, 2H, CH2), 5.25 (brs, 1H, NH), 7.32 (d, 2H, J = 5.5Hz, H-2), 7.57 (d, 2H, J = 5.5 Hz, H-3).
2-((1H-benzo[d]imidazol-2-yl)thio) acetohydrazide (4)[23].
A mixture of 3 (2.36g, 0.01 mole), hydrazine hydrate (1.5 g, 0.03 mole) and ethanol (40 ml) was heated under reflux for 3h (TLC). The product was filtered off, recystallized from ethanol to yield white powder in 92% yield, m.p.143-145°C Rf = 0.33 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 2.11 (brs, 2H, NH2), 4.50 (s, 2H, CH2), 5.35 (brs, 1H, NH), 7.27 (d, 2H, J= 5.5 Hz, Ar-H), 7.55 (d, 2H, J= 5.5 Hz, Ar-H), 8.35 (brs, 1H, NH).
Reaction of (4) with Different Sugars to Afford the Corresponding Sugar Hydrazones 5( a-d )
To solution of 4 (10 mmol) in absolute ethanol, different sugars (10 mmol) were added and then glacial acetic acid (1 ml) was added to the reaction mixture which was refluxed for 10h (TLC). The solvent was evaporated or concentrated under reduced pressure and the product was filtered off to afford 5(a-d) (83 – 88%) yields.
L-(-)–Arabinose(2-mercaptobenzimidazole-1-1-yl) acetohydrazide (5a)
Yellow powder (83%), m.p.182-184°C. Rf = 0.65 (5% MeOH in CH2Cl2), 1H NMR (DMSO-d6): δ= 3.12-3.85 (m, 5H, H-2′, H-3′, H-4′, H-5′, H-5′′), 2.75 (brs, 1H, OH), 3.52-3.61 (brs, 3H, 3xOH), 4.05 (s, 2H, CH2), 4.95 (brs, 1H, NH), 6.95-7.32 (m, 4H, Ar-H), 7.40 (brs, 1H, NH), 7.55 (s, 1H, CH).
D-(-)–Glucose(2-mercaptobenzimidazole-1-1-yl) acetohydrazide (5b)
White powder (85%), m.p.202-204°C. Rf = 0.65 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 3.14-3.92 (m, 5H, H-3′, H-4′, H-5′, H-6′, H-6′′), 3.10 (m, 1H, H-2′), 3.42 (brs, 1H, OH), 3.55 (brs, 2H, 2xOH), 3.62 (brs, 2H, 2xOH), 4.15 (s, 2H, CH2), 4.90 (brs, 1H, NH), 6.90 (brs, 1H, NH), 6.93-7.35 (m, 4H, Ar-H), 7.55 (s, 1H, CH).
D-(-)–Mannose(2-mercaptobenzimidazole-1-1-yl) acetohydrazide (5c)
White powder (90%), m.p.196-198 °C. Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ = 3.16-3.95 (m, 5H, H-3′, H-4′, H-5′, H-6′, H-6′′), 3.12 (m, 1H, H-2′), 3.45 (brs, 1H, OH), 3.60 (brs, 2H, 2xOH), 3.62 (brs, 2H, 2xOH), 4.15 (s, 2H, CH2), 4.90 (brs, 1H, NH), 6.93 (brs, 1H, NH), 6.95-7.40 (m, 4H, Ar-H), 7.55 (s, 1H, CH).
D-(-)–Galactose(2-mercaptobenzimidazole-1-1-yl) acetohydrazide (5d)
White powder (90%), m.p.174-176 °C. Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 3.13-3.94 (m, 5H, H-3′, H-4′, H-5′, H-6′, H-6′′), 3.13 (m, 1H, H-2′), 3.44 (brs, 1H, OH), 3.59 (brs, 2H, 2xOH), 3.61 (brs, 2H, 2xOH), 4.15 (s, 2H, CH2), 4.92 (brs, 1H, NH), 6.92 (brs, 1H, NH), 6.94-7.38 (m, 4H, Ar-H), 7.60 (s, 1H, CH).
General Procedure for Preparation of Sugar of tetra-O-acetyl- and penta-O-acetyl(2-mercaptobenzimidazol-1-yl)acetylhydrazones 6 (a- d)
A mixture of the sugar hydrazones 5 (a-d) (10 mmol), was dissolved in 30 ml of pyridine and then ( 15 mmol ) of acetic anhydride was added. The reaction was stirred at room temperature overnight (TLC). The mixture was cooled and poured on crushed ice.The precipitate was filtered off and dried to give 6 (a- d) in 75-92% yields.
2,3,4,5-Tetra-O-acetyl-L-(-)-arabinose(2-mercaptobenzimidazol-1-yl) acetylhydrazone (6a)
Yellow gum (75%), Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 1.99, 2.01, 2.10, 2.21 (4s, 12H, 4xCH3CO), 4.15 (s, 2H, CH2), 4.30,4.41 (2m, 2H, H-5′, H-5′′), 4.60 (m, 1H, H-2′), 5.06 ( brs, 1H, NH ), 5.10 (m, 1H, H-4′), 5.12 (m, 1H, H-3′), 7.12 ( brs, 1H, NH ), 7.52 (d, 1H, J=2.5 Hz, H-1′), 7.25-7.62 (m,4H, Ar-H).
2,3,4,5-Tetra-O-acetyl-D-(+)-Glucose(2-mercaptobenzimidazol-1-yl)acetylhydrazone (6b)
Yellow gum (80%), Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 1.97, 2.03, 2.04, 2.06, 2.25 (5s, 15H, 5xCH3CO), 4.13(s, 2H, CH2), 4.25,4.47 (2m, 2H, H-6′,H-6′′), 4.60 (m, 1H, H-2′), 5.00 (m, 1H, H-3′), 5.05 (brs, 1H, NH), 5.10 (m, 1H, H-4′), 5.12 (m, 1H, H-5′), 7.12 ( brs, 1H, NH), 7.25-7.58 (m, 4H, Ar-H), 7.60 (d, 1H, J=2.5 Hz, H-1′).
2,3,4,5-Tetra-O-acetyl-D-(+)-Mannose(2-mercaptobenzimidazol-1-yl)acetylhydrazone (6c)
Yellow gum (85%), Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 2.11, 2.18, 2.20, 2.22, 2.26 (5s, 15H, 5xCH3CO), 4.11(s, 2H, CH2), 4.29,4.47 (2m, 2H, H-6′,H-6′′), 4.57 (m, 1H, H-2′), 5.04 (m, 1H, H-3′), 5.09 (brs, 1H, NH), 5.13 (m, 1H, H-4′), 5.20 (m, 1H, H-5′), 7.19 ( brs, 1H, NH), 7.29-7.59 (m, 4H, Ar-H), 7.65 (d, 1H, J=2.5 Hz, H-1′).
2,3,4,5-Tetra-O-acetyl-D-(+)-Galactose(2-mercaptobenzimidazol-1-yl)acetylhydrazone (6d)
Brown gum (92%), Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 2.16, 2.21, 2.24, 2.26, 2.29 (5s, 15H, 5xCH3CO), 4.11(s, 2H, CH2), 4.27,4.49 (2m, 2H, H-6′,H-6′′), 4.58 (m, 1H, H-2′), 5.03 (m, 1H, H-3′), 5.07 (brs, 1H, NH), 5.12 (m, 1H, H-4′), 5.16 (m, 1H, H-5′), 7.17 ( brs, 1H, NH), 7.27-7.57 (m, 4H, Ar-H), 7.63 (d, 1H, J=2.5 Hz, H-1′).
General Procedure for the Synthesis of 4-acetyl-5-(tetra-and penta-O-acetylalditolyl)-2-(2-mercaptobenzimidazol-1-yl)-1, 3, 4-oxadiazoline 7 (a-d)
A solution of sugar hydrazones 5(a-d) (0. 01 mol) in acetic anhydride (10 ml) was heated at 90°C for 3 h. The resulting solution was poured onto crushed-ice and the product that separated out was filtered off, washed with water, and then dried. The products were recrystallized from methanol to give 7(a-d) in 70-78 yields.
4-Acetyl-5-(1,2,3,4-tetra-O-acetyl-L-arabinotetritolyl)-2-(2mercaptobenzimidazol-1-yl)-1,3,4-oxadiazoline (7a)
Brown oil (70%), Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 2.03, 2.05, 2.07, 2.10, 2.21 (5s, 15H, 5xCH3CO), 4.40 (s, 2H, CH2), 4.12, 4.45 (2m, 2H, H-4′,H-4′′), 5.05 (brs, 1H, NH), 5.14 (m, 1H, H-3′), 5.19 (m, 1H, H-2′), 5.89 (dd, 1H, J=3.2, 6.2 Hz, H-1′), 5.89 (d, 1H, J=8.8 Hz, oxadiazoline H-5), 7.24-7.59 (m, 4H, Ar-H).
4-Acetyl-5-(1,2,3,4,5-penta-O-acetyl-D-glucopentitolyl)-2(2-methylbenzimidazol-1-yl)-1,3,4-oxadiazoline (7b)
Brown oil (72%), Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ=2.04, 2.07, 2.09, 2.12, 2.22, 2.34 (6s, 18H, 6xCH3CO), 4.60 (s, 2H, CH2), 4.27,4.48 (2m, 2H, H-5′,H-5′′), 5.10 (brs, 1H, NH),, 5.15 (m, 1H, H-4′), 5.21 (m, 1H, H-3′), 5.28 (m, 1H, H-2′), 5.32 (dd, 1H, J=3.2, 6.2 Hz, H-1′),5.93 (d, 1H, J=8.8 Hz, oxadiazoline H-6), 7.25-7.60 (m, 4H, Ar-H).
4-Acetyl-5-(1,2,3,4,5-penta-O-acetyl-D-glucopentitolyl)-2(2-methylbenzimidazol-1-yl)-1,3,4-oxadiazoline (7c)
Brown oil (73%), Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 1.97, 1.99, 2.03, 2.06, 2.10, 2.32 (6s, 18H, 6xCH3CO), 4.60 (s, 2H, CH2), 4.24,4.46 (2m, 2H, H-5′,H-5′′), 5.12 (brs, 1H, NH),, 5.16 (m, 1H, H-4′), 5.22 (m, 1H, H-3′), 5.26 (m, 1H, H-2′), 5.34 (dd, 1H, J=3.2, 6.2 Hz, H-1′),5.94 (d, 1H, J=8.8 Hz, oxadiazoline H-6), 7.22-7.52 (m, 4H, Ar-H).
4-Acetyl-5-(1,2,3,4,5-penta-O-acetyl-D-glucopentitolyl)-2(2-methylbenzimidazol-1-yl)-1,3,4-oxadiazoline (7d)
Brown oil (78%), Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ=1.95, 1.97, 1.99, 2.04, 2.07, 2.34 (6s, 18H, 6xCH3CO), 4.58 (s, 2H, CH2), 4.25,4.47 (2m, 2H, H-5′,H-5′′), 5.11 (brs, 1H, NH),, 5.17 (m, 1H, H-4′), 5.21 (m, 1H, H-3′), 5.26 (m, 1H, H-2′), 5.32 (dd, 1H, J=3.2, 6.2 Hz, H-1′),5.90 (d, 1H, J=8.8 Hz, oxadiazoline H-6), 7.20-7.560 (m, 4H, Ar-H).
General Procedures for the Reaction of (4) with Aromatic Aldehydes to Afford Schiff′s bases 8(a-d)
A solution of 4 (0.01 mol), an aromatic aldehyde (0.01 mol) in abs. ethanol (30 ml) and glacial acetic acid (1 ml) was refluxed for 6-8 h (TLC). The solvent was evaporated under reduced pressure and the residue was filtered off and recrystallized from ethanol to afford 8(a-d) in 88-94 % yields.
2-((1H-benzo[d]imidazol-2-yl)thio)-N’-benzylideneacetohydrazide (8a)
Yellow gum (88%), Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ=4.18 (s, 2H, CH2), 5.55 (brs, 1H, NH), 7.23-7.85 (m, 9H, Ar-H),8.13 (s, 1H, CH), 8.45 (brs, 1H, NH).
2-((1H-benzo[d]imidazol-2-yl)thio)-N’-(2-hydroxybenzylidene)acetohydrazide (8b)
Yellow gum (90%), Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ=4.15 (s, 2H, CH2), 5.10 (brs, 1H, NH), 5.34 (s, 1H, OH), 7.05-7.65 (m, 8H, Ar-H), 8.00 (brs, 1H, NH), 8.95(s, 1H, CH).
2-((1H-benzo[d]imidazol-2-yl)thio)-N’-(furan-2-ylmethylene)acetohydrazide (8c)
White crystals (92%), m.p. 160-164°C. Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 4.20 (s, 2H, CH2), 5.15 (brs, 1H, NH), 6.50-7.80 (m, 7H, Ar-H), 7.95 (brs, 1H, NH), 8.42 (s, 1H, CH).
2-((1H-benzo[d]imidazol-2-yl)thio)-N’-(4-(dimethylamino)benzylidene)acetohydrazide (8d)
White crystals (94%), m.p. 158-160°C. Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 3.10 (s, 6H, 2CH3), 4.18 (s, 2H, CH2), 5.11 (brs, 1H, NH), 6.58-7.64 (m, 9H, Ar-H), 8.05 (brs, 1H, NH), 8.37 (s, 1H, CH).
General Procedure for Preparation of Oxadiazoline Derivatives 9 (a-d)
A mixture of the shiff bases 8(a-d) (10 mmol), were dissolved in acetic anhydride (10 ml) and the reaction mixture was refluxed at 100 ºC 5-7h (TLC). The mixture was onto crushed-ice and the product that separated out was filtered off, washed with water, and then dried. The products were recrystallized from methanol to give 9(a-d) in 80-88 yields.
1-(5-(((1H-benzo[d]imidazol-2-yl)thio)methyl)-2-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethanone (9a)
Yellow powder (80%) m.p. 152-154°C. Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 2.10 (s, 3H, COCH3), 4.50 (s, 2H, CH2), 5.13 (brs, 1H, NH), 6.55 (s, 1H, CH) 7.24-7.57 (m, 9H, Ar-H).
1-(5-(((1H-benzo[d]imidazol-2-yl)thio)methyl)-2-(2-hydroxyphenyl)-1,3,4-oxadiazol-3(2H)-yl)ethanone (9b)
Yellow powder (84%) m.p. 136-138°C. Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 2.12 (s, 3H, COCH3), 4.35 (s, 2H, CH2), 5.11(brs, 1H, NH), 5.37 (s, 1H, OH), 6.95 (s, 1H, CH) 6.85-7.56 (m, 8H, Ar-H).
1-(5-(((1H-benzo[d]imidazol-2-yl)thio)methyl)-2-(furan-2-yl)-1,3,4-oxadiazol-3(2H)-yl)ethanone (9c)
Yellow powder (85%) m.p. 142-144ºC. Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 2.09 (s, 3H, COCH3), 4.40 (s, 2H, CH2), 5.14(brs, 1H, NH), 6.87 (s, 1H, CH), 6.35-7.69 (m, 7H, Ar-H).
1-(5-(((1H-benzo[d]imidazol-2-yl)thio)methyl)-2-(4-(dimethylamino)phenyl)-1,3,4-oxadiazol-3(2H)-yl)ethanone (9d)
White crystals (88%), m.p. 170-172ºC. Rf = 0.72 (5% MeOH in CH2Cl2). 1H NMR (DMSO-d6): δ= 3.12 (s, 6H, 2CH3), 2.10 (s, 3h, COCH3), 4.50 (s, 2H, CH2), 5.14 (brs, 1H, NH), 6.65 (s, 1H, CH) 6.27-7.66 (m, 8H, Ar-H).
Materials and Methods
Antibacterial Sensitivity Test
Microbiological investigation hole well method the investigated isolates of bacteria were seeded in tubes with nutrient broth (NB) two different bacteria species were used Escherichia coli (E.coli) ,Staphylococcus epidermidis (S. epidermidis) and Staphylococcus aureus (S.aureus). antimicrobial activity of chemical compounds was evaluated by Hole well method , Briefly ,inoculum containing approx. cell density (1.5 * 108 CFU/ml ) was spread on Mueller-Hinton agar (MHA) plates the holes diameter( 0.5 cm ) were done in the cool medium after that 50µ from different concentration of compounds 10,20 and 30 was applied using a micropipette .After incubation for 24h in an incubator at 37ºC for bacteria the inhibition zone diameters were measured and expressed in cm. The previous procedure was repeated with 30 μg/disc of tetracycline and with ampicillin at 10 μg/disc [24].
Microbiological Investigation of Compounds
Antibacterial of compounds are carried out against the (Gram –ve) as E.coli and ( Gram +ve) as S.aureus and (S. epidermidis) , the antimicrobial activity was estimated based on size of inhibition zone around dishes against Gram+v the 7a (figure 1) 5a ,5c,9a( figures 2,3,4) and 9b are varied degree of inhibitory effect against S.aureus and 8a,bc and 8a varied degree of inhibitory effect against S.epidermidis ,also in Gram-v the 8b,6c,6a,8a,7a( figure 5),5c and 9b are varied degree of inhibitory effect against E.coli. The antibacterial activities of compounds against E. coli ,S. aureus and S.epidermids tested and compared to known antibiotics (Tables).
The results showed that increasing the zone of inhibition in compared with known antibiotic Amoxicillin and tetracycline .the data showed in table1.
Table 1: Antibacterial activity of different synthesized compounds.
| E.coli | S.e | S.aureus | |||||||
| Zone of inhibition (mm) | |||||||||
| Synthesized | 30 mg/mL | 60 mg/mL | 90 mg/mL | 30 mg/mL | 60 mg/mL | 90 mg/mL | 30 mg/mL | 60 mg/mL | 90 mg/mL |
| compounds | |||||||||
| 8b | 40 | 45 | 50 | 17 | 20 | 25 | – | – | – |
| 6c | 13 | 15 | 22 | 12 | 15 | 17 | – | – | – |
| 6a | 13 | 17 | 20 | 10 | 14 | 15 | – | – | – |
| 8a | 15 | 18 | 22 | 8 | 17 | 20 | – | – | – |
| 7a | 11 | 12 | 20 | 8 | 10 | 12 | – | – | – |
| 5c | 15 | 20 | 22 | – | – | – | 12 | 15 | 17 |
| 5a | 11 | 13 | 17 | – | – | – | 18 | 22 | 23 |
| 7a | 8 | 11 | 13 | – | – | – | 11 | 13 | 14 |
| 9b | 18 | 19 | 21 | – | – | – | 18 | 20 | 22 |
| Amoxicillin | 18 | 10 | 8 | ||||||
| tetracycline 30 | 13 | 8 | 6 | ||||||
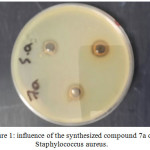 |
Figure 1: influence of the synthesized compound 7a on Staphylococcus aureus. Click here to View figure |
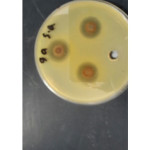 |
Figure 2: influence of the synthesized compound 9a on Staphylococcus aureus. Click here to View figure |
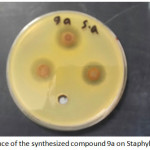 |
Figure 3: influence of the synthesized compound 9a on Staphylococcus aureus. Click here to View figure |
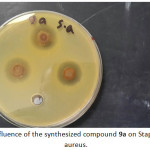 |
Figure 4: influence of the synthesized compound 9a on Staphylococcus aureus. Click here to View figure |
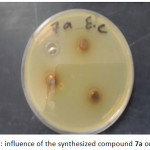 |
Figure 5: influence of the synthesized compound 7a on E.coli. Click here to View figure |
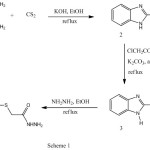 |
Scheme 1 Click here to View scheme |
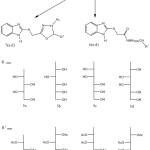 |
Scheme 2 Click here to View scheme |
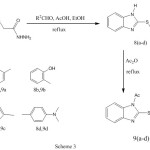 |
Scheme 3 Click here to View scheme |
Conclusion
In this research the 2-mercaptobenzimidazole derivatives were synthized and then were coupled with different sugars ( arabinose, glucose, mannose and arabinose ) and aromatic aldehydes to give the corresponding sugar hydrazones and arylidene derivatives respectively. The synthized compounds were showed high to moderate activity against the (Gram –ve) as E.coli and ( Gram +ve) as S.aureus and (S. epidermidis).
Acknowledgments
The authors are pleased to acknowledge Taif University for providing the facilities for the research.
References
- Vikash, K. C.; Devender, P.; Pushpendra, K.; Vijay, Y. Der Pharmacia Lettre. 2016, 8(3),127-134
- Najim, A. M.; Nadhir, N. A.; Layla, J. A.; Sadiq, J. B.; Christophe, P. Z. Naturforsch. 2011, 66b, 953-960
- Chavan, B. B.; Chitte, P. D.; Choudhary, N. P.; Albhar, K. G.; Hukkeri, V. I. International Journal of Scientific Research and Reviews. 2012, 1(3), 22-30
- Kiran, M. K.; Sagar, A. J.; Pramod, B. P.; Vikas, R. D.; Shitalkumar, S. P. Der Pharma Chemica. 2016, 8(4), 1-5
- Hamiduzzaman, M. d.; Mannan, S. J.; Dey, A.; Abdur Rahman, S. M. Der Pharmacia Lettre. 2014, 6(1), 47-53
- Saumya, G.; Surya, P. G.; Neeraj, U.; Gopal, G. Journal of Drug Design and Medicinal Chemistry. 2015, 1(2), 12-1
- Anandarajagopal, K.; Ravi, N. T.; Venkateshan, N.; Pooshan, G. V.; Promwichit, P. J. Chem. Pharm. Res. 2010, 2(3), 230-236
- Kiran, M. K, Sagar, A. J.; Pramod, B. P.; Vikas R. D.; Shitalkumar, S. P. Der Pharma Chemica. 2016, 8(4), 1-5
- Saumya, G.; Surya, P. G.; Neeraj, U.; Gopal, G. Journal of Drug Design and Medicinal Chemistry. 2015, 1(2), 12-16
- Kumar, D. M.; Dubey, P. K. Indian journal of chemistry. 2012, 51 B, 1619-1622
- Heralagi, R. V.; Jayaveera, K. N.; Shivkumar, B. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2012, 3(2), 407-414
- Neha, P. V.; Suganthi, V.; Gowri, S. Der Pharma Chemica. 2013, 5(2),139-143
- Gigani, Y.; Jadhav, S. Int J Pharm Bio Sci. 2010, 1(4), 281-286
- Kaur, P.; Wakode, S. R. International Journal of Science and Research. 2016, 5(3), 762-772
- Zygmunt, K.; Jacqueline, A. U.; Peter, U.; Agata, G.; Bohdan, S.; Agnieszka, L. Acta Biochemica Polonica. 2002, 49(1), 185-195
- Gangula, M. R.; Yellu, N. R.; Baru, V. K. International journal of applied biology and pharmaceutical technology. 2013, 4(1), 38-46
- Sahu, S. K.; Banerjee, M.; Samantray, A.; Behera, C.; azam, M. A. Tropical Journal of Pharmaceutical Research. 2008, 7(2), 961-968
- Ramanpreet, W.; edaitullah, M. d.; Syeda, F. N.; Khalid, I.; Lamba, H. S. International Journal of Research in Pharmacy and Chemistry. 2011, 1(3), 565-574
- Singh, G.; Kaur, M.; Chander, M.; Rayat, B. International Research Journal of Pharmacy. 2013, 4(1), 82-87
CrossRef - Suraj, B. A.; Deshp, M. N.; Kolhatkar, D. G. Int J Pharm Bio Sci. 2012, 3(2), 6-11
- Reddy, C. M.; Jayakar, B.; Srinivasan, R. Int J Pharm Bio Sci. 2010, 1(4), 81-86
- Ansari, K. F, Lal, C. European Journal of Medicinal Chemistry. 2009, 44(10), 4028–4033
CrossRef - Kus, C.; Ayhan-Kılcıgil, G.; zbey, S. O.; Kaynak, F.; Kaya, M.; Can-Eke, B. Bioorganic & Medicinal Chemistry. 2008, 16(8), 4294–4303
- Majali, I. S.; Oran, S. A.; Khleifat, K. M.; Qaralleh, H.; Rayyans, W. A.; Althunibat, O. Y. African Journal of Microbiology Research. 2015, 9(51), 2410-2414
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









