Synthesis of new -1,3,4-Thiadiazoles Substituted with Oxazepine and Benzoxazepine Moieties
Faez Abdul-Hussein Alrammahi1, Qasim Mehdi Ismael1 and Zeid Hassan Abood2
1Department of Chemistry, Faculty of Education for Girls , University of Kufa, Najaf, Iraq.
2Department of Chemistry, College of Science, University of Kerbala, kerbala, Iraq.
Corresponding Author E-mail: faez.alrammahi@uokufa.edu.iq
DOI : http://dx.doi.org/10.13005/ojc/330536
2-amino-5-mercapto-1,3,4-thiadiazole 1 was introduced in condensation reaction with terephthaldehyde to yield bis-imine derivative 2. Compound 1 was also converted to the corresponding diazonium salt which was introduced in coupling reactions with alkaline olution of 2-hydroxybenzaldehyde and 4-hydroxybenzaldehyde as coupling reagents to give azo derivatives 4a and 4b containing aldehyde group, respectively. The resulting aldehydes 4a and 4b were then introduced in condensation reactions with 2-amino-5-mercapto-1,3,4-thiadiazole 1 to obtain the imines 5a and 5b respectively. The resulting imines 2, 5a and 5b were treated with both maleic and phthalic anhydrides, respectively, under (2+5) cycloaddition conditions afforded eight new bis-1,3,4-thiadiazoles substituted with 1,3-oxazepine and 1,3-benzoxazepine moieties (3a, 3b) and (6a-d)respectively. The new synthesized thiadiazoles probably have some biological, pharmaceutical and medicinal applications.
KEYWORDS:1,3,4-Thiadiazoles; 2-Amino-5-Mercapto-1,3,4-Thiadiazole; Imines; 1,3-Oxazepines; 1,3-benzoxazepines; Heterocyclic; Bis-diazonium; Terephthaldehyde
Download this article as:| Copy the following to cite this article: Alrammahi A. A. H, Ismael Q. M, Abood Z. H. Synthesis of new -1,3,4-Thiadiazoles Substituted with Oxazepine and Benzoxazepine Moieties. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Alrammahi A. A. H, Ismael Q. M, Abood Z. H. Synthesis of new -1,3,4-Thiadiazoles Substituted with Oxazepine and Benzoxazepine Moieties. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=39361 |
Introduction
Thiadiazoles are clear to yellowish liquids which are soluble in alcohol, ether and slightly soluble in water; they are starting material for numerous chemical compounds including sulphur drugs(1). Thiadiazoles are easily metabolized by biochemical reactions and they are non-carcinogenic in nature(2). Thiadiazoles and their derivatives exhibit wide range of pharmacological activities such as antimicrobial activity(3), antidepressant, cardiotonic(4), antibacterial activity against Klebsiella pneumonia(5), antitubercular(6,7), anticonvulsant(8), antileshmanial , analgesic(9), antiinflammtory(10), anticancer(11), phosphodiesterase inhibitors(12) and effect on Tyrosinase enzyme(13). This diversity of biological activity may be due to the presence of -N=C-S moity(14,15). There are four isomers of thiadiazole , among these four isomers 1,3,4-thiadiazole is the most thermally stable; which is only isomer doesn´t contain any sulphur- nitrogen bond(16). 1,3,4-thiadiazole relatively stable in aqueous acid solutions but the nucleus can undergo ring cleavage by aqueous base solutions(17).
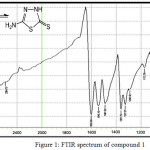 |
Figure 1: FTIR spectrum of compound 1 Click here to View figure |
Heterocyclic seven-membered ring constitutes the core or a key fragment of a number of bioactive compounds including isolated from natural products, oxazepine derivatives were found to exhibit a vast variety of biological activities(18) and found to be a vital moiety in many psychoactive pharmaceuticals(19). Thus, in this article, we reported here the synthesis of 1,3,4-thiadiazole derivatives containing biologically active oxazepine and benzoxazepine moieties, which might have some biological activity.
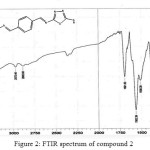 |
Figure 2: FTIR spectrum of compound 2 Click here to View figure |
Experimental
General
The chemicals used were purchased from Merck, BDH, sigma Aldrich and CDH and were used without further purification. Silica TLC plates were used with an aluminum backing (0.2 mm, 60 F254). The progress of reactions were monitored by TLC and visualized by development of the TLC plates with iodine vapor. Melting points were determined on an Electro thermal Stuart SMP 30 capillary melting point apparatus. Infrared spectra were recorded on SHIMADZU FTIR-8400S Infrared Spectrophotometer as potassium bromide discs. 1H NMR spectra were collected on NMR spectrometer, Broker 2009 spectrometer at 400 MHz in DMSO-d6 as solvent and TMS as an internal standard at Kashan University, Iran. Elemental Analysis (CHNS) was carried out with Perkin Elmer 300A Elemental Analyzer at Kashan University, Iran. Azoaldehyde derivatives 4a and 4b were prepared following the method described by Acton (20).
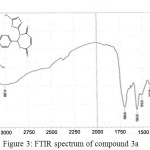 |
Figure 3: FTIR spectrum of compound 3a Click here to View figure |
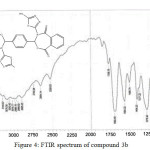 |
Figure 4: FTIR spectrum of compound 3b Click here to View figure |
Chemical Methods
Synthesis of 5,5′-(((1E,1’E)-1,4-phenylenebis(methanylidene))bis(azanylylidene))bis(1,3,4-thiadiazole-2-thiol) 2
Terephthaldehyde (0.67 g, 5 mmol) was dissolved in (35 mL) of absolute ethanol, then 2-amino-5-mercapto-1,3,4-thiadiazole 1 (1.33 g , 10 mmol) was added. The reaction mixture was refluxed with stirring on a water bath at 65oC for 12 h and monitored by TLC. The mixture was then allowed to cool down to room temperature , the colored precipitate was filtered and recrystallized from ethanol: IR (cm-1): 3194br (νN-H, thione form and νC-H, benzene, vib. coupling), 2972 and 2885 (νN-H, intramolecularly hydrogen bonded, thione form), 1691 (νC=N, imine), 1562 and 1508 (νC=C, benzene and νC=N, thiadiazole, vib. coupling), 1051 (νC=S, thione form), 750 (δo.o.p. C-H, benzene); 1H NMR: δ = 2.5 (DMSO solvent), 3.4 (H2O in DMSO), 7.09–8.17 (m, 4H, Ar-H ), 8.8 (s, 2H, 2×CH=N, imine), 10.09 (s, 2H, 2×N-H, thione forms), 13.17 (s, 2H, 2×S-H, thiol forms) (21) .
 |
Figure 5: FTIR spectrum of compound 4a Click here to View figure |
General Procedure for the Synthesis of Oxazepine and Benzoxazepine Derivatives (3a, 3b)
A mixture of bisimine derivative 2 (0.364 g, 1 mmol) and maleic or phthalic anhydride (2 mmol) in dry benzene (20 mL) was refluxed on a water bath at 70ºC for 20 h and monitored by TLC. The mixtures were then allowed to cool down to room temperature, the colored precipitates were filtered, dried and recrystallized from ethanol.
 |
Figure 6: FTIR spectrum of compound 4b Click here to View figure |
2,2′-(1,4-phenylene)bis(3-(5-mercapto-1,3,4-thadiazol-2-yl)-2,3-dihydro-1,3-oxazepine-4,7-dione) 3a
IR (cm-1): 3134 (νN-H, thione form), 3007 (νC-H, benzene), 1699 (νC=O, O=C-O and O=C-N, oxazepine, vib. coupling), 1564 and 1510 (νC=C, benzene and νC=N, thiadiazole, vib. coupling), 1053 (νC=S, thione form), 752 (δo.o.p. C-H, benzene); 1H NMR: d (ppm) = 6.2 and 6.6 (ss, 4H, 4×olefinic =CH, oxazepine), 7.10–8.16 (m, 6H, Ar–H and C–H, oxazepine), 10.1 (s, 2H, 2×N–H, thione forms, 13.2 (s, 2H, 2×S–H, thiol forms (21) . The singlet signals around 2.5 ppm and 3.3 ppm attributed to DMSO and absorbed H2O in DMSO, respectively. Anal. Calcd. for C20H12N6O6S4: C, 42.85; H, 2.16; N, 14.99; S, 22.88 Found C, 42.52; H, 2.19; N, 15.22; S, 22.51.
 |
Figure 7: FTIR spectrum of compound 5a Click here to View figure |
3,3′-(1,4-phenylene)bis(4-(5-mercapto-1,3,4-thadiazol-2-yl)-3,4-dihydrobenzo[e][1,3]oxazepine-1,5-dione) 3b
IR (cm-1): 3144 (νN-H, thione form), 3076 and 3016 (νC-H, benzene), 2989 and 2895 (νN-H, intramolecularly hydrogen bonded, thione form), 2740 (νC-H, oxazepine), 2654 and 2526 (νS-H, thiol form), 1695 (νC=O, O=C-O and O=C-N, oxazepine, vib. coupling), 1564 and 1498 (νC=C, benzene and νC=N, thiadiazole, vib. coupling), 1062 (νC=S, thione form), 798 and 742 (δo.o.p. C-H, benzene); 1H NMR: d (ppm) = 7.08–8.14 (m, 14H, Ar–H and C–H, oxazepine), 10.1 (s, 2H, 2×N–H, thione forms), 13.16 (s, 2H, 2×S–H, thiol forms). The singlet signals around 2.5 ppm and 3.3 ppm attributed to DMSO and absorbed H2O in DMSO, respectively. Anal. Calcd. for C28H16N6O6S4: C, 50.90; H, 2.44; N, 12.72; S, 19.41 Found C, 51.23; H, 2.62; N, 12.43; S, 19.55.
 |
Figure 8: FTIR spectrum of compound 5b Click here to View figure |
Synthesis of Azo-aldehyde Derivatives (4a, 4b)
Were prepared following the method described by Acton (20).
2-hydroxy-5-((5-mercapto-1,3,4-thiadiazol-yl)diazenyl)benzaldehyde 4a
IR (cm-1): 3281br (νO-H), 3093 (νN-H, thione form and ν C-H, benzene, vib. coupling), 2953 (νN-H, intramolecularly hydrogen bonded, thione form), 1629 (νC=O, aldehyde), 1556 and 1498 (νC=C, benzene and ν C=N, thiadiazole, vib. coupling), 1037 (νC=S, thione form), 833 (δo.o.p. C-H, benzene) ); 1H NMR: d (ppm) = 6.93–7.81 (m, 3H, Ar–H), 10.2 (s, 1H, O=CH, aldehyde), 10.7 (s, 1H, O-H), 13.1 (s, 1H, S–H) (20) . he singlet signals around 2.48 ppm and 3.85 ppm attributed to DMSO and absorbed H2O in DMSO, respectively.
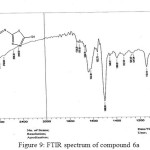 |
Figure 9: FTIR spectrum of compound 6a Click here to View figure |
4-hydroxy-5-((5-mercapto-1,3,4-thiadiazol-yl)diazenyl)benzaldehyde 4b
IR (cm-1): 3284br (νO-H), 3095br (νN-H, thione form and νC-H, benzene, vib. coupling), 2962 and 2899 (νN-H, intramolecularly hydrogen bonded, thione form), 2829 and 2700 (νC-H, aldehyde), 1627 (νC=O, aldehyde), 1552 and 1498 (νC=C, benzene and νC=N, thiadiazole, vib. coupling), 1039 (νC=S, thione form), 837 (δo.o.p. C-H, benzene); 1H NMR: d (ppm) = 6.90–7.74 (m, 3H, Ar–H), 8.8 (s, 1H, O-H), 9.7 (s, 1H, O=CH, aldehyde), 12.34 (s, 1H, S–H)
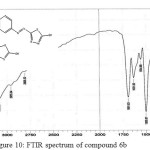 |
Figure 10: FTIR spectrum of compound 6b Click here to View figure |
General Procedure for the Synthesis of Azoimine Derivatives (5a, 5b)
Azoaldehyde derivatives 4a or 4b (0.665 g, 2.5 mmol) was dissolved in (30 mL) of absolute ethanol, then 2-amino-5-mercapto-1,3,4-thiadiazole 1 (0.3325 g , 2.5 mmol) was added. The reaction mixtures were refluxed with stirring on a water bath at 65oC for 10 h and monitored by TLC. The mixtures were then allowed to cool down to room temperature , the colored precipitates were filtered and recrystallized from ethanol.
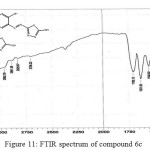 |
Figure 11: FTIR spectrum of compound 6c Click here to View figure |
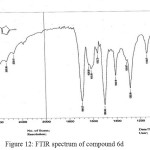 |
Figure 12: FTIR spectrum of compound 6d Click here to View figure |
4-((E)-(5-mercapto-1,3,4-thiadiazol-2-yl)diazenyl-2-((E)-((5-mercapto-1,3,4-thiadiazole-2-yl)imino)methyl)phenol 5a
IR (cm-1): 3269br (νO-H), 3109 (νN-H, thione form and νC-H, benzene, vib. coupling), 2943 (νN-H, intramolecularly hydrogen bonded, thione form), 1620 (νC=N, imine), 1554 and 1498 (νC=C, benzene and νC=N, thiadiazole, vib. coupling), 1051 (νC=S, thione form), 752 (δo.o.p. C-H, benzene); 1H NMR: d (ppm) = 7.00–7.77 (m, 4H, Ar–H and HC=N, imine), 10.2 (s, 1H, O-H), 13.16 (s, 2H, 2×S–H) (20).
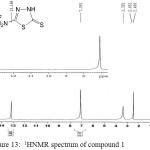 |
Figure 13: 1HNMR spectrum of compound 1 Click here to View figure |
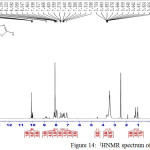 |
Figure 14: 1HNMR spectrum of compound 2 Click here to View figure |
2-((E)-(5-mercapto-1,3,4-thiadiazol-2-yl)diazenyl-4-((E)-((5-mercapto-1,3,4-thiadiazole-2-yl)imino)methyl)phenol 5b
IR (cm-1): 3267br (νO-H), 3099br (νN-H, thione form and νC-H, benzene, vib. coupling), 2949 and 2802 (νN-H, intramolecularly hydrogen bonded, thione form), 2584 (νS-H), 1610 (νC=N, imine), 1554 and 1502 (νC=C, benzene and νC=N, thiadiazole, vib. coupling), 1051 (νC=S, thione form), 752 and 715 (δo.o.p. C-H, benzene); 1H NMR: d (ppm) = 1H NMR: d (ppm) = 6.9–7.7 (m, 3H, Ar–H), 8.8 (s, 1H, N=CH, imine), 9.6 and 9.7 (ss, 2H, 2×N-H, thione forms), 10.5 (s, 1H, O-H), 13.4 (s, 2H, 2×S–H, thiol forms).
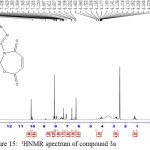 |
Figure 15: 1HNMR spectrum of compound 3a Click here to View figure |
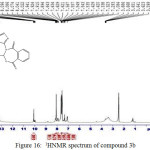 |
Figure 16: 1HNMR spectrum of compound 3b Click here to View figure |
General Procedure for the Synthesis of Oxazepine and Benzoxazepine Derivatives 6a-d
A mixture of azoimine derivatives 5a or 5b (0.381 g, 1 mmol) and maleic or phthalic anhydride (1 mmol) in dry benzene (20 mL) was refluxed on a water bath at 70ºC for 24 h and monitored by TLC. The mixtures were then allowed to cool down to room temperature, the colored precipitates were filtered, dried and recrystallized from ethanol.
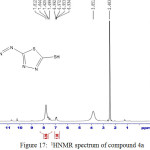 |
Figure 17: 1HNMR spectrum of compound 4a Click here to View figure |
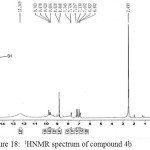 |
Figure 18: 1HNMR spectrum of compound 4b Click here to View figure |
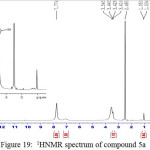 |
Figure 19: 1HNMR spectrum of compound 5a Click here to View figure |
2-(2-hydroxy-5-((5-mercapto-1,3,4-thiadiazole-2-yl)diazenyl)phenyl)-3-(5-mercapto-1,3,4-thiadiazole-2-yl)-2,3-dihydro-1,3-oxazepine-4,7-dione 6a
IR (cm-1): 3280br (νO-H), 3105br (νN-H, thione form and νC-H, benzene, vib. coupling), 2949 and 2791 (νN-H, intramolecularly hydrogen bonded, thione form), 2708 (νC-H, oxazepine), 2536 (νS-H), 1640 (νC=O, O=C-O and O=C-N, oxazepine, vib. coupling), 1552 and 1498 (νC=C, benzene and νC=N, thiadiazole, vib. coupling), 1055 (νC=S, thione form), 756 (δo.o.p. C-H, benzene); 1H NMR: d (ppm) = 6.2 and 6.6 (ss, 2H, 2×olefinic =CH, oxazepine), 7.13–8.19 (m, 4H, Ar–H and C–H, oxazepine), 10.35 (s, 1H, O-H), 13.16 (s, 2H, 2×S–H). The singlet signals around 2.47 ppm and 3.49 ppm attributed to DMSO and absorbed H2O in DMSO. Anal. Calcd. for C15H9N7O4S4: C, 37.57; H, 1.89; N, 20.45; S, 26.75 Found C, 37.28; H, 2.09; N, 20.22; S, 26.47.
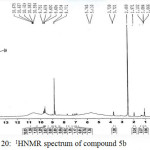 |
Figure 20: 1HNMR spectrum of compound 5b Click here to View figure |
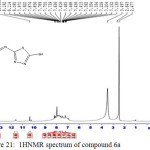 |
Figure 21: 1HNMR spectrum of compound 6a Click here to View figure |
(E)-3-(2-hydroxy-3-((5-mercapto-1,3,4-thiadiazole-2-yl)diazenyl)phenyl)-4-(5-mercapto-1,3,4-thiadiazole-2-yl)-3,4-dihydrobenzo[e][1,3]oxazepine-1,5-dione 6b
IR (cm-1): 3267br (νO-H), 3086br (νN-H, thione form and νC-H, benzene, vib. coupling), 2955 and 2806 (νN-H, intramolecularly hydrogen bonded, thione form), 2700 (νC-H, oxazepine), 2525 (νS-H), 1691 (νC=O, O=C-O, oxazepine), 1631 ( O=C-N, oxazepine), 1554 and 1500 (νC=C, benzene and νC=N, thiadiazole, vib. coupling), 1062 (νC=S, thione form), 798 (δo.o.p. C-H, benzene); 1H NMR: d (ppm) = 7.34–7.64 (m, 8H, Ar–H and C–H, oxazepine), 7.79 (s, 1H, O-H), 13.16 (s, 2H, 2×S–H).. Anal. Calcd. for C19H11N7O4S4: C, 43.09; H, 2.09; N, 18.51; S, 24.22 Found C, 43.36; H, 2.22; N, 18.21; S, 24.54.
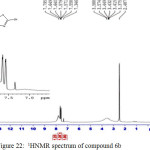 |
Figure 22: 1HNMR spectrum of compound 6b Click here to View figure |
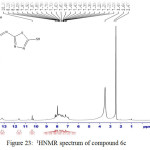 |
Figure 23: 1HNMR spectrum of compound 6c |
2-(4-hydroxy-3-((5-mercapto-1,3,4-thiadiazole-2-yl)diazenyl)phenyl)-3-(5-mercapto-1,3,4-thiadiazole-2-yl)-2,3-dihydro-1,3-oxazepine-4,7-dione 6c
IR (cm-1): 3281br (νO-H), 3078 (νN-H, thione form and νC-H, benzene, vib. coupling), 2962 and 2891 (νN-H, intramolecularly hydrogen bonded, thione form), 2829 (νC-H, oxazepine), 1705 (νC=O, O=C-O, oxazepine), 1631 ( O=C-N, oxazepine), 1550 and 1500 (νC=C, benzene and νC=N, thiadiazole, vib. coupling), 1039 (νC=S, thione form), 866 (δo.o.p. C-H, benzene); 1H NMR: d (ppm) = 6.24 and 6.59 (ss, 2H, 2×olefinic =CH, oxazepine), 7.13–7.32 (m, 4H, Ar–H and C–H, oxazepine), 8.78 (s, 1H, O-H), 9.5 (s, 2H, 2×S–H). Anal. Calcd. For C15H9N7O4S4: C, 37.57; H, 1.89; N, 20.45; S, 26.75 Found C, 37.32; H, 2.06; N, 20.14; S, 27.07.
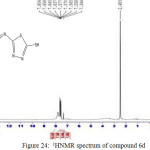 |
Figure 24: 1HNMR spectrum of compound 6d Click here to View figure |
(E)-3-(4-hydroxy-3-((5-mercapto-1,3,4-thiadiazole-2-yl)diazenyl)phenyl)-4-(5-mercapto-1,3,4-thiadiazole-2-yl)-3,4-dihydrobenzo[e][1,3]oxazepine-1,5-dione 6d
IR (cm-1): 3277br (νO-H), 3091 (νN-H, thione form and νC-H, benzene, vib. coupling), 2954 (νN-H, intramolecularly hydrogen bonded, thione form), 2655 (νC-H, oxazepine), 2530 (νS-H), 1691 (νC=O, O=C-O, oxazepine), 1629 ( O=C-N, oxazepine), 1552 and 1500 (νC=C, benzene and νC=N, thiadiazole, vib. coupling), 1070 and 1055 (νC=S, thione forms), 802 and 740 (δo.o.p. C-H, benzene); 1H NMR: d (ppm) = 7.34–7.65 (m, 8H, Ar–H and C–H, oxazepine), 7.80 (s, 1H, O-H), 13.16 (s, 2H, 2×S–H). Anal. Calcd. For C19H11N7O4S4: C, 43.09; H, 2.09; N, 18.51; S, 24.22 Found C, 42.88; H, 2.15; N, 18.77; S, 24.48.
Results and Discussion
Chemistry
The precursor bisimine 2 was synthesized by reacting terephthaldehyde with 2-amino-5-mercapto-1,3,4-thiadiazole 1 in absolute ethanol. Compound 2 was reacted with maleic and phthalic anhydrides to give the bis-1,3-oxazepine and bis-1,3-benzoxazepine derivatives of 1,3,4-thiadiazole 3a and 3b respectively in Scheme (1). The proposed mechanism for the addition of cyclic anhydride to imine was shown in Scheme (2).
The chemical structures of these newly oxazepines were confirmed by IR, 1H NMR spectral measurements and (CHNS) elemental analysis and were in good agreement with the proposed structures.
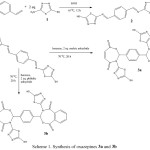 |
Scheme 1: Synthesis of oxazepines 3a and 3b Click here to View scheme |
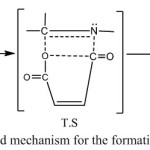 |
Scheme 2: Proposed mechanism for the formation of oxazepine ring Click here to View scheme |
The IR spectrum of bis-imine 2 showed the stretching absorption band of the (C=N) function at 1691 cm-1, while the absorption bands due to (NH2) group at 3336 and 3267 cm-1 have disappeared.The broad absorption band at 3194 attributed to the (N-H)str. in thione form. The stretching band of (C=S) function in thione form appeared as strong band at 1051 cm-1. The IR spectra of compounds 3a and 3b indicated the absence of (C=N) absorption band and the appearance of (C=O)str. for oxazepine ring at 1699 and 1695 cm-1, respectively.
The 1H NMR spectrum of bisimine 2 showed the (HC=N) protons as a singlet at δ 8.8 ppm, the (N-H) protons of thione forms appeared as a singlet at 10.09 ppm, the (Ar-H) protons at δ 7.09 –8.17 ppm. Moreover, the spectrum showed the (SH) protons as a singlet at 13.17 ppm. (21).
The 1H NMR spectra of oxazepine compounds 3a and 3b showed the disappearance of the (CH=N) protons at 8.8 ppm, the thiolic (S-H) protons appeared as a singlet at δ 13.2 and 13.16 ppm, respectively. The (N-H) protons for thione forms as a singlet at 10.1. The signals of aromatic protons (Ar–H) and (C–H) protons of oxazepine rings appeared at δ 7.08-8.16 ppm. Moreover, the olefinic (=CH) protons of the oxazepine rings in compound 3a appeared as singlet at 6.2 and 6.6 ppm.
The initiators azoaldehydes 4a and 4b were synthesized by reacting the diazonium salt of 2-amino-5-mercapto-1,3,4-thiadiazole 1 with alkaline solutions of 2-hydroxybenzaldehyde and 4-hydroxybenzaldehyde respectively using the method described by acton(20). The resulting aldehydes 4a and 4b were condensed with 2-amino-5-mercapto-1,3,4-thiadiazole 1 in absolute ethanol to give the azoimine derivatives 5a and 5b respectively. The resulting imines 5a and 5b were allowed to react with maleic and phthalic anhydrides leading to the formation of oxazepine-1,3,4-thiadiazole derivatives 6a-d respectively (Scheme 3).
The structures of the compounds synthesized were deduced by IR, 1H NMR spectral measurements and (CHNS) elemental analysis and were in good agreement with the proposed structures.
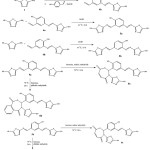 |
Scheme 3: Synthesis of oxazepines 6a-b
|
The IR spectra of azoaldehde derivatives 4a and 4b indicated the absence of the doublet band at 3336 and 3267 cm-1 for (-NH2)str. and appearance of broad band at 3281 and 3284 cm-1 assigned to (O-H)str., the absorption band at 1629 and 1627 cm-1 belong to the aldehydic (C=O)str., respectively. The IR spectra of imine derivatives 5a and 5b showed the disappearance of the absorption bands at 1629 and 1627 cm-1 for aldehydic (C=O)str., also disappearing the doublet band for (-NH2)str. in 2-amino-5-mercapto-1,3,4-thiadiazole at 3336 and 3267 cm-1, while the absorption bands attributed to (C=N)str. appeared at 1620 and 1610 cm-1, respectively. In the IR spectra of oxazepine compounds 6a-d, the stretching absorption band due to the stretching of (C=O, oxazepine) was found at 1640, (1691 and 1631), (1705 and 1631), (1691 and 1629) cm-1, respectively, while the absorption bands due to (C=N)str. at 1620 and 1610 cm-1 have disappeared.
The 1H NMR spectra of azoaldehyde compounds 4a and 4b showed the (S-H) proton as a singlet at δ 13.1 and 12.34 ppm (20) .the (O-H) proton appeared as a singlet at 10.7 and 8.8 ppm, the (HC=O) proton as a singlet at δ 10.2 and 9.7 ppm, , the (Ar-H) protons at δ 6.90 –7.81 ppm.
The 1H NMR spectra of imine compounds 5a and 5b showed the (S-H) proton as a singlet at δ 13.6 and 13.4 ppm, the (O-H) proton appeared as a singlet at 10.2 and 10.5 ppm, the (HC=N) proton as a singlet at δ 7.77 and 8.8 ppm, , the (Ar-H) protons at δ 6.90 –7.77 ppm.
The 1H NMR spectra of oxazepine compounds 6a-d showed the disappearance of the (CH=N) protons at 7.77 and 8.8 ppm, the (S-H) proton appeared as a singlet at δ 13.6, 13.6, 9.5 and 13.16 ppm, respectively. The (O-H) proton as a singlet at 10.35, 7.79, 8.78 and 7.80 ppm. The signals of aromatic protons (Ar–H) and (C–H) proton of oxazepine rings appeared at δ 7.13-8.19 ppm. Moreover, the olefinic (=CH) protons of the oxazepine ring in compounds 6a and 6c appeared as a singlets at (6.2, 6.6) and (6.24, 6.59) ppm, respectively.
The structures of the compounds synthesized were proven by IR, 1H NMR spectral measurements and (CHNS) elemental analysis and were in good agreement with the proposed structures.
The IR spectrum of bis-azoaldehde derivative 7 showed disappearance of the sharp doublet band for (-NH2)str. at the range (3400-3250) cm-1 and appearance of band at 3365 cm-1 assigned to (O-H)str., the strong absorption band at 1662 cm-1 due to the aldehydic (C=O)str. The IR spectrum of bis-imine derivative 8 indicated the disappearance of the absorption band at 1662 cm-1 for aldehydic (C=O)str., also disappearing the doublet band for (-NH2)str. in 2-amino-5-mercapto-1,3,4-thiadiazole at 3336 and 3267 cm-1, while the absorption band attributed to (C=N)str. appeared at 1602 cm-1. The IR spectra of oxazepine compounds 9a and 9b showed the stretching absorption band due to (C=O, oxazepine) at (1714, 1662) cm-1 and 1689 cm-1 respectively, while the absorption band due to (C=N) at 1602 cm-1 has disappeared.
The biological activity of the synthesized oxazepines of 1,3,4-thiadiazole 3a, 3b and 6a-d will be measured in subsequent study.
FT-IR spectra of compounds 1, 2, 3a, 3b, 4a, 4b, 5a, 5b and 6a-d
1H NMR spectra of compounds 1, 2, 3a, 3b, 4a, 4b, 5a, 5b and 6a-d
Table 1: Physical Properties of the Synthesized Compounds
|
Product |
Physical state |
Rf (developer) |
m.p. (oC) |
Yield (%) |
|
2 |
Light yellow solid |
0.64 (Toluene/ EtOH, 7:3) |
186-188 |
79 |
|
3a |
Light yellow solid |
0.81 (Toluene/ EtOH, 7:3) |
148-150 |
77 |
|
3b |
Light yellow solid |
0.78 (Toluene/ EtOH, 7:3) |
170-172 |
79 |
|
4a |
Dark orange solid |
0.54 (Toluene/ EtOH, 7:3) |
181-183 |
61 |
|
4b |
Dark orange solid |
0.49 (Toluene/ EtOH, 7:3) |
129-131 |
55 |
|
5a |
Orange solid |
0.57 (Toluene/ EtOH, 7:3) |
179-181 |
70 |
|
5b |
Orange solid |
0.73 (Toluene/ EtOH, 7:3) |
178-180 |
71 |
|
6a |
Dark yellow solid |
0.8 (Toluene/ EtOH, 7:3) |
163-165 |
78 |
|
6b |
Dark yellow solid |
0.75 (Toluene/ EtOH, 7:3) |
161-163 |
80 |
|
6c |
Orange solid |
0.56 (Toluene/ EtOH, 7:3) |
123-125 |
79 |
|
6d |
Dark yellow solid |
0.72 (Toluene/ EtOH, 7:3) |
156-158 |
81 |
Acknowledgements
We are highly thankful to staff of the central laboratory, University of Kashan, Iran for their significant assistance in 1H NMR and Elemental Analysis measurements of the synthesized compounds. Supporting for Synthesis of New -1,3,4-Thiadiazoles Substituted with Oxazepine and benzoxazepine Moieties
References
- S. Jalhan, A. Jidal, A. Gupta and Hemraj, Asian journal of pharmaceutical and clinical Reseach, 5, 199 (2012).
- M.M. Raj, H.V. Patel, L.M. Raj and N.K. Patel, IJPCBS, 3, 814 (2013).
- S. Adhikari, S.B. Bari, A. Samanta, Journal of Applied Chemical Research, 8, 31 (2014).
- Society of Ecological Chemistry and Engineering, Influence of 1,3,4-thiadiazole derivatives on the biological activity of the selected environmental bacteria, Opole University, Vol.18, No.12, pp. 1691-1692 (2011)
- N. Aggarwal, R. Kumar, P. Dureja and J.M. Khurana, Chem Bio Drug Des, 79, 384 (2012).
CrossRef - M. Amir, A. Kumar, I. Ali and S.A. khan, Indian J. Chem., 48B, 1288 (2009).
- S.H. Joshi and M.K. ThaKer, Indian J. Chem., 44B, 410 (2005).
- B. Ahmed and M.d. Yusuf, Indian J. Chem., 49B, 241 (2010).
- D.E. Abdel Rahman and K.O. Mohamed, Der Pharma Chemica, 6, 323 (2014).
- F. A. Hassan, IJRPC, 2, 58 (2012).
- A.S. Mayhoub, L. Marler, T.P. Kondratyuk, E.J. Park, J.M. Pezzuto and M. Cushman, Bioorg. Med. Chem., 20, 2427 (2012).
CrossRef - F. Vergne, P. Bernardelli, E. Lorthiois, N. Pham, E. Proust, C. Oliveira, A. Mafroud and F. Royer, Bioorg. Med. Chem. Lett., 14, 4607 (2004).
CrossRef - M.M. El-Sadek, S.Y. Hassan, H.E. Abdelwahab and G.A. Yacout, Molecules, 17, 8378 (2012).
CrossRef - E. Oruc, S. Rollas, F. Kandemirli, N. Shavets and A. Dimoglo, J. Med. Chem., 47, 6760 (2004).
CrossRef - S. Jaiswal and S. Sigh, International Journal of Engineering Research and General Science 2, 167 (2014).
- S. Alrammahi and F. A. Alrammahi, International Journal of Advanced Multidisciplinary Research, 1, 38 (2014).
- Y. Hu, C.Y. Li, X.M. Wang, Y.H. Yang and H.L. Zhu, ACS Publications, p. 27,2013).
- F. Hajishaabanha and A. Shaabani, RSC adv., 4, 46844 (2014).
CrossRef - J.F. Liegeois, F.A. Rogister, J. Bruhwyler, J. Damas, T.P. Nguyen, M.O. Inarejos, E.M. Chleide, M.G. Mercier and J.E. Delarge, J. Med. Chem., 37, 519 (1994).
CrossRef - Q.A. Acton, Azo Compounds: Advances in Research and Application, Scholarly Paper Edition, Atlanta, p.42 (2011).
- N.A. Salih, Turk J. Chem. 32,pp 229-235(2008)

This work is licensed under a Creative Commons Attribution 4.0 International License.









