Synthesis and Antioxidant Ability of Some New 6-amino-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl) Derivatives Bearing 2,6-Dimethoxy-4-(methoxymethyl)Phenol Moiety
Raied Mustafa Shakir, Khalid Fahad Ali and Dhuha Faruk Hussain
Department of Chemistry, Ibn Al-haitham, University of Baghdad, Baghdad 61023, Iraq.
Corresponding Author E-mail: raiedalsayab@yahoo.co.uk
DOI : http://dx.doi.org/10.13005/ojc/330543
Compound 4-(((6-amino-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)methoxy) methyl)-2,6-dimethoxyphenol (6) was synthesized by multi steps. The corresponding acetonitrile thioalkyl (7) was cyclized by refluxing with acetic acid to afford 4-(((6-amino-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxy phenol (8). Two new series of 4-(((6-(3-(4-aryl)thioureido)-7H-[1,2,4]triazolo [3,4-b][1,3,4] thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxyphenol (9a-c) and of 4-(((6-(substitutedbenzamido)7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)methoxy) methyl)-2,6-dimethoxyphenol (10a-c) were synthesized as new derivatives for fused 1,2,4-trizaole- thiadiazine (8). The antioxidants of newly compounds were evaluated by DPPH and FRAP assays. Compound 9b showed significant antioxidant ability in both assays (higher than ascorbic acid) as well compound 6, 8 and 10a-c showed antioxidant higher than BHT.
KEYWORDS:2,6-dimethoxyphenol; 6-amino thiadiazine; fused 1,2,4-triazole; antioxidant
Download this article as:| Copy the following to cite this article: Shakir R. M, Ali K. F, Hussain D. F. Synthesis and Antioxidant Ability of Some New 6-amino-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl) Derivatives Bearing 2,6-Dimethoxy-4-(methoxymethyl)Phenol Moiety. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Shakir R. M, Ali K. F, Hussain D. F. Synthesis and Antioxidant Ability of Some New 6-amino-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl) Derivatives Bearing 2,6-Dimethoxy-4-(methoxymethyl)Phenol Moiety. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=39108 |
Introduction
The free radicals are one of the most important factors that cusses critical disadvantage to biomolecules, for instance, proteins, carbohydrates, lipids, and DNA 1. Such this disadvantage considered as source of many infirmity such as inflammatory2 and cancers infirmity 3, degenerative infirmity 4 and Chronic infirmity. For that, the antioxidants compounds considered one of the significant materials due to their capacity to terminate the free radicals or diminish the oxidation effect. Furthermore, many anti-inflammatory and antinecrotic medications reported it possess antioxidant ability besides their therapeutic properties5. Commonly, the free radical scavenging compounds endue protons and transform to extra stable free radicals. This stability rises with the presence of delocalization and rises the antioxidant ability6,7 . Otherwise, the presence of multiple hydroxyl groups or presence full conjugation( π system) in structure and steric hindrance these factors has positive influence on the antioxidant ability8-10.
Fused rings with 1,2,4-triazole exhibited intensive interests for their wide biological activity. For instance, antimicrobials activity11, antitumor12,13, herbicidal activity14, anti-inflammatory15, antifungal activity16, anti-HIV-1 activity17 and antioxidant activity18. Moreover, fused 1,2,4-triazole –thiadiazine exhibited wide biological activity19-21. In the other hand, the 2,6-dimethoxyphenol derivatives, has been reported exhibited interesting antioxidant properties 22,23. In this work we presented synthesis some new 6-amino-7h-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl) derivatives bearing 2,6-dimethoxy-4-(methoxymethyl)phenol moiety. And evaluated their antioxidant ability by DPPH and FRAP assays.
Material and Method
Chemistry
The melting point was detected by open capillary tube method utilizing OMEGA MPS10, apparatus and it is uncorrected. The purities of synthesized compounds were checked with a thin layer chromatography (Silica gel TLC) plates and the spots were visualized either with UV lights or iodine vapors. The FTIR spectrua were obtained with Perkin Elmer 400 Fourier Transform Infrared (FTIR) Spectrometer. 1D NMR spectra were recorded by Bruker AVN 400 MHz. CDCl3 andDMSO-d6 were utilized as a solvent with TMS as internal standard, measurements. The accurate mass spectra were recorded by utilizing a Finnigan TSQ7000 for HREIMs (NUS, Singapore). The FRAP Assay and DPPH assay (as antioxidant assay) were recording by using ultraviolet spectroscopy Power Wave X340,BIO-TEK instrument.
ethyl 2-((3,5-dimethoxy-4-((trimethylsilyl)oxy)benzyl)oxy)acetate.
Ethyl bromoacetate (5.1g, 30 mmol) was added drop wise with in 1h to stirring suspension of (3,5-dimethoxy-4-((trimethylsilyl)oxy)phenyl)methanol (7.69 g, 30 mmol) in 50 mL DCM and 20 ml of 15% sodium hydroxide in the presence of tetrahexylammonium bromide (THAB) (0.65 g, 5% mmol). After complete the addition the mixture was left under stirring for 18 h at room temperature. The mixture transferred to separation funnel to extract the organic layer. The organic layer was with distilled water (10 mL×2). The organic layer was evaporated under reduced pressure after it dried under sodium sulfate. The product was purified by column chromatography utility hexane: ethyl acetate (6:1) to afford yellow oil which is solidified at 0 ̊C to give white solid. Yield 74%, Mp 7-9 °C. FTIR (liquid film) υ max; 3046 (CHAr), 2983, 2867 (CHaliphatic), 1724 (C=O), 1601, 1584 (C=C), 1208 (Ar-O-C), 870 (Si-CH3) cm-1. 1H NMR 400 MHz, CDCl3): δ 0.16 (9H, s, Si(CH3)3, 1.34 (3H, t, J 7.2, CH3),.3.85 (6H, s, 2×OCH3), 4.24 (2H, q, J 7.3, OCH2CH3), 4.40 (2H, s, OCH2CO2Et), 4.78 (2H, s, CH2OCH2), 6.61 (2H, s, H-3). 13C NMR (100MHz.CDCl3) δ; -0.057 (3C, Si(CH3)3), 15.22 (1C, CH3) ,57.09 (2C,2× OCH3), 59.35 (1C, OCH2CH3), 65.18 (1C, OCH2CO2Et), 71.07(1C, CH2OCH2), 109.11(2C, C3). 127.94 (1C, C1), 132.54 (1C, C-4), 150.91 (1C, C-2), 171.04 (1C, C=O). HREIMs m/z = 342.1499 [M·+] (calc. for C16H26O6Si, 342.1499).
2-((4-hydroxy-3,5-dimethoxybenzyl)oxy)acetic acid.
A suspension of ethyl 2-((3,5-dimethoxy-4-((trimethylsilyl)oxy)benzyl)oxy)acetate. (3) (7.5 g, 21 mmol) in 25 mL of 50 % acetic acid: THF (2:1) was refluxed overnight. After cooling the PH of the mixture was adjusted to 8-9 by saturated solution of sodium hydrogen carbonate, and then extracted by chloroform. The organic layer was neglected. The aqueous layer was acidified using 5% hydrochloric acid. The precipitated was filtrated, washed with distilled water and dried to obtain white amorphous solid. Yield 69.2%. Mp 128-130 °C. FTIR (KBr) υ max; 3455 (OH ), 3033 (CHAr), 2977, 2854 (CHaliphatic), 1687 ( C=O), 1595, 1483 (C=C), 1198 (Ar-O-C) cm-1. 1H NMR 400 MHz, DMSO-d6): δ 3.87 (6H, s, 2×OCH3), 4.34 (2H, s, OCH2COOH), 4.81(2H, s, CH2OCH2), 6.65 (2H, s, H-3), 9.24 (bs, 1H, OH), 12.76 (1H, bs, COOH). 13C NMR (100MHz, DMSO-d6) δ; 56.83 (2C,2× OCH3), 64.77 (1C, OCH2COEt), 70.88 (1C, CH2OCH2), 108.94 (2C, C-3), 132.60 (1C, C-4), 137.94 (1C, C-1), 151.03 (1C, C-2), 178.25 (1C, C=O). HREIMs m/z = 242.0786 [M·+] (calc. for C11H14O6, 242.0790).
2-((4-hydroxy-3,5-dimethoxybenzyl)oxy)acetohydrazide
Thionylchloride (5 mL) was added droop wise to 2-((4-hydroxy-3,5-dimethoxy benzyl)oxy)acetic acid. (4) (3.6 g, 14.86 mmol). The stirring mixture was refluxed for 3h. The remains of thionyl chloride was evaporated under reduce pressure. The resulting acid chloride (without further purification) was transferred to an addition funnel with 10 mL dry benzene. 5 mL of hydrazine hydrate (98%) in 10 mL dried benzene was added into a two neck flask that equipped with a condenser. After that the addition funnel fixed onto the flask and the solution of acid chloride was added dropwise at 0°C. After completion the addition, the mixture incubated for 1 h with stirring at an ambient temperature, then for further it was refluxed 3h .The solvent was removed under reduce pressure. The crude product collected and washed with water then recrystallized from aqueous ethanol to afford white needle crystal. Yield 82% . Mp 103-106°C. FTIR (KBr) υmax; 3464 (OH ), 3318, 3205, 3197 (NHNH2(, 3042 (CHAr), 2984, 2870 (CHaliphatic), 1661 ( C=O), 1603, 1485 (C=C), 1206 (Ar-O-C) cm-1. 1H NMR (400 MHz, DMSO-d6): δ 3.89 (6H, s, 2×OCH3), 4.37 (2H, s, OCH2CO), 4.79 (2H, s, CH2OCH2), 4.54 (1H, bs, NH2), 6.66 (2H, s, H-3), 8.76 (1H, bs. CONH), 9.32 (1H, bs, OH).13C NMR (100MHz, DMSO-d6) δ; 56.91 (2C,2× OCH3), 68.22 (1C, OCH2CONH), 71.27 (1C, CH2OCH2), 109.09 (2C,C-3). 132.67 (1C, C-4), 137.83 (1C, C-1), 150.95 (1C, C-2), 166.05 (1C, C=O). HREIMs m/z = 256.1054 [M·+] (calc. for C11H16N2O5, 256.1059).
4-amino-3-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)-1H-1,2,4-triazole-5(4H)-thione
To stirred a solution of 2-((4-hydroxy-3,5-dimethoxybenzyl)oxy)acetohydrazide (3.0 g, 11.70 mmol) in 12 mL absolute ethanol Carbon disulphide (1.35 g, 17.5 mmol) and potassium hydroxide (065 g, 11.7 mmol) were added at ambient temperature. The mixture allowed to stirred for 24 h, and then 20 mL dry diethyl ether was added and then stirring for further 2 h. The precipitated was collected by filtration then washed with dry diethyl ether. The product was dried at 70 °C to give white solid potassium 2-(2-((4-hydroxy-3,5-dimethoxybenzyl)oxy) acetyl)hydrazinecarbodithioate salt (3.69 g, 11.15 mmol). The product was dissolved in 10 mL of hydrazine hydrate 80%. And heated under refluxed for 7 h. after cooling it was poured into crushed ice. The pH of solution was adjusted to 5-6 by utilizing 5% HCl. The precipitate collected and washed with water, dried then recrystallized from methanol to obtain 2.25 g (65%) of white precipitate, m.p.142-144°C. FTIR (KBr) υmax 3511 (OH phenol), 3411, 3304 and 3169 (NH2, NH), 3025 (CHAr), 2986 -2877 (CHaliphatic), 1630 (C=N), 1594-1477 (C=C), 1358 (C-N), 1231(C=S), 1202(Ar-O-C), cm-1. 1H-NMR (400MHz, DMSO-d6) δ; 3.63 (2H, s, CH2OCH2), 3.84(6H, s, 2× OCH3), 4.55 (2H, s, CH2OCH2), 5.98 (2H, bs, NH2), 6.69 (2H, s, H-3), 9.30 (1H, bs, OH), 11.07 (1H, bs, NH). 13C-NMR (100 MHz, DMSO-d6) δ; 57.13 (2C, 2×OCH3), 67.83 (1C, CH2OCH2), 71.95 (1C, CH2OCH2), 110.73 (2C, C-3), 132.85 (1C, C-4), 138.54 (1C, C-1), 149.59 (1C, C=N), 152.11 (2C, C-2), 169.08 (1C, C=S). HREIMs m/z = 312.0888 [M·+] (calc. for C12H16N4O4S, 312.0892).
2-((4-amino-5-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)-4H-1,2,4-triazol-3-yl)thio)acetonitrile
Chloroacetonitrile (0.5 g, 6.62 mmol) was added in small portions to a stirred suspension of compound 6 (2.06 g, 6.62 mmol) in anhydrous acetone and anhydrous potassium carbonate (0.91g, 6.62 mmol). The mixture was left to stand overnight with stirring at ambient temperature. The solvent was evaporated and the residue extracted with 30 mL chloroform. It was dried under anhydrous sodium sulfate and evaporated under reduced pressure. The crud product was recrystallized from acetonitrile to afford off white precipitate. Yield 84%, Mp 68-70°C. FTIR (KBr) υmax 3556 (OH phenol), 3411, 3304 (NH2), 3031 (CHAr), 2985 -2865 (CHaliphatic), 2249 (CN), 1626(C=N), 1598,1475 (C=C), 1347 (C-N), 1192 (Ar-O-C), cm-1. 1H-NMR (400MHz, DMSO-d6) δ; 3.84 (6H, s, 2× OCH3), 4.30 (2H, s, CH2CN), 4.55 (2H,s, CH2OCH2), 4.60 (2H, s, CH2OCH2), 6.02 (2H, bs, NH2), 6.65 (2H, s, H-3), 9.30 (1H, bs, OH). 13C-NMR (100 MHz, DMSO-d6) δ; 18.33 (1C, CH2CN) 56.81 (2C, 2×OCH3), 65.44 (1C, CH2OCH2), 70.58 (1C, CH2OCH2), 110.73 (2C, C-3), 115.0 (1C, CN), 132.49 (1C, C-4), 137.98 (1C, C-1), 151.89 (2C, C-2), 152.02 (1C, C=N), 157.18 (1C, C=N). HREIMs m/z = 351.0998 [M·+] (calc. for C14H17N5O4S, 351.1001).
4-(((6-amino-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxyphenol
2-((4-amino-5-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)-4H-1,2,4-triazol-3-yl) thio)acetonitrile (1.80 g, 5.12 mmol) in 10 mL acetic acid was heated under reflux for 24 h after cooling the precipitated was collected and washed with 5% sodium hydrogen carbonate solution then distilled water . The crude product was recrystallized from aqueous DMF to obtain pale yellow precipitated. Yield 59%. Mp 114-116 °C. FTIR (KBr) υmax; 3542 (OH phenol), 3343, 3219 (NH2), 3028 (CHAr), 2988, 2873 (CHaliphatic), 1618 (C=N), 1595, 1477 (C=C), 1342 (C-N), 1197 (Ar-O-C), cm-1. . 1H-NMR (400MHz, DMSO-d6) δ; 3.85(2H, s, 2× OCH3), 4.41(2H, s, H-9), 4.58 (2H, s, CH2OCH2), 4.68 (2H, s, CH2OCH2), 6.65 (6H, s, H-3), 7.34 (2H, bs, NH2), 9.07 (1H, bs, OH). 13C-NMR (100 MHz, DMSO-d6) δ; 37.57(1C, C-9) 57.21 (2C, 2×OCH3), 60.62 (1C, CH2OCH2), 70.49 (1C, CH2OCH2), 110.24(2C, C-3), 131.87 (1C, C-4), 138.25 (1C,C-1), 151.73 (2C, C-2), 154.48 (1C, C=N), 157.16 (1C, C=N),157.70 (1C, C=N). HREIMs m/z = 351.0995 [M·+] (calc. for C14H17N5O4S, 351.1001).
General synthesis of 4-(((6-(3-(4-aryl)thioureido)-7H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxyphenol 9a-c
aryl is othiocyanate (0.6 mmole) was added with small portion to hot solution of 4-(((6-amino-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl) methoxy)methyl)-2,6-dimethoxy phenol (0.21g, 0.6 mmol) in absolute ethanol (10 mL). The mixture heated at 50 C for 2h. Upon cooling the precipitate filtrated and washed with cold ethanol. The crud material was recrystallized from suitable solvent.
4-(((6-(3-(4-chlorophenyl)thioureido)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxyphenol 9a
The crude solid recrystallized from aqueous methanol to obtain white precipitate 83% Yield. Mp 167-169°C. FTIR (KBr) υmax 3496 (OH phenol), 3230 (NH), 3032 (CHAr), 2990, 2868 (CHaliphatic), 1622 (C=N), 1596,1481 (C=C), 1340 (C-N), 1257 (C=S), 1197 (Ar-O-C) cm-1. 1H-NMR (400MHz, DMSO-d6) δ; 3.83(6H, s, 2× OCH3), 4.38 (2H, s, H-9), 4.61 (2H,s, CH2OCH2), 4.71 (2H, s, CH2OCH2), 6.64 (2H, s, H-3), 6.81 (2H, d, J 8.21, H-13), 7.34 (2H, d, J 8.20, H-14), 8.84 (1H, bs, NH), 8.90 (1H, bs, NH), 9.11 (1H, bs, OH). 13C-NMR (100 MHz, DMSO-d6) δ; 37.45(1C, C-9) 58.01 (2C, 2×OCH3), 60.67 (1C, CH2OCH2), 70.55 (1C, CH2OCH2), 109.94 ( 2C, C-3), 128.83 (2C, C-14), 130.52 (2C, C-13), 132.08 (1C, C-4), 134.42(1C, C-15), 137.15 (1C, C-12), 138.77 (1C, C-1), 151.68 (2C, C-2), 154.53 (1C, C=N), 157.20 (1C, C=N), 157.751(1C, C=N). 181.92 (1C, C=S) HREIMs m/z = 520.0752 [M·+] (calc. for C21H21ClN6O4S2, 520.0754).
4-(((6-(3-(4-methylphenyl)thioureido)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxyphenol 9b
The crude product recrystallized from methanol to obtain white precipitate 85% Yield. Mp 134-136 °C. FTIR (KBr) υmax 3528(OH phenol), 3235 (NH), 3019 (CHAr), 2996, 2872 (CHaliphatic), 1629 (C=N), 1595, 1488 (C=C), 1341 (C-N), 1250 (C=S), 1210 (Ar-O-C) cm-1. 1H-NMR (400MHz, DMSO-d6) δ; 2.21 (3H, s, CH3), 3.86 (6H, s, 2× OCH3), 4.41 (2H, s, H-9), 4.64 (2H,s, CH2OCH2), 4.70 (2H, s, CH2OCH2), 6.56 (2H, d, J 7.78, H-13), 6.68 (2H, s, H-3), 7.23 (2H, d, J 8.02, H-14), 8.86 (1H, bs, NH), 8.93 (1H, bs, NH), 9.35 (1H, bs, OH). 13C-NMR (100 MHz, DMSO-d6) δ; 19.80 (1C, CH3), 37.59 (1C, C-9) 57.89 (2C, 2×OCH3), 60.83 (1C, CH2OCH2), 71.05 (1C, CH2OCH2), 111.13 (2C, C-3), 127.92 (2C, C-13), 129.74 (2C, C-14), 132.33 (1C, C-4), 134.88 (1C, C-12), 137.15 (1C, C-15), 139.08. (1C, C-1), 150.67 (2C, C-2), 155.51 (1C, C=N), 157.35 (1C, C=N), 157.76 (1C, C=N). 181.69 (1C, C=S) HREIMs m/z = 500.1294 [M·+] (calc. for C22H24N6O4S2, 500.1300).
4-(((6-(3-(4-methoxylphenyl)thioureido)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxyphenol 9c
The crude product recrystallized from ethanol to obtain white precipitate 78% Yield. Mp 128-130 °C. FTIR (KBr) υmax 3547 (OH phenol), 3231 (NH), 3049 (CHAr), 2994, 2875 (CHaliphatic), 1630 (C=N), 1601, 1486 (C=C), 1338 (C-N), 1247 (C=S), 1212 (Ar-O-C) cm-1. 1H-NMR (400MHz, DMSO-d6) δ; 3.84 (9H, s, 3× OCH3), 4.39 (2H, s, H-9), 4.62 (2H, s, CH2OCH2), 4.72 (2H, s, CH2OCH2), 6.37 (2H, d, J 8.2, H-13), 6.68 (2H, s, H-3), 6.98 (2H, d, J 8.12, H-14), 8.85 (1H, bs, NH), 8.91 (1H, bs, NH), 9.14 (1H, bs, OH). 13C-NMR (100 MHz, DMSO-d6) δ; 37.59 (1C, C-9), 56.92 (1C, OCH3), 57.83 (2C, 2×OCH3), 61.54 (1C, CH2OCH2), 70.95 (1C, CH2OCH2), 111.13 (2C, C-3), 121.32 (2C, C-14), 127.44 (2C, C-13), 132.51 (1C, C-4), 133.66 (1C, C12), 138.58 (1C, C1), 150.67 (2C, C-2), 151.04 (1C, C-15), 155.69 (1C, C=N), 156.75 (1C, C=N), 158.22 (1C, C=N). 181.09 (1C, C=S) HREIMs m/z = 516.1247 [M·+] (calc. for C22H24N6O5S2, 516.1250).
General synthesis of 4-(((6-(substitutedbenzamido)7H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxyphenol 10a-c
To a stirring suspension 4-(((6-amino-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl) methoxy)methyl)-2,6-dimethoxy phenol ( 0.21g, 0.6 mmol) in 15mL dry pyridine at 0C, aryloxy acid chloride (0.65 mmole) was added dropwise in period 30 mint through additional funnel. After completion the addition the mixture left to stirring at ambient temperature overnight. The mixture poured in to 50 mL crushed ice then extracted from DCM. The organic layer washed by 2% hydrochloric acid then distilled water after that dried over sodium sulfate. The crude product was purified by column chromatography.
4-(((6-(4-hydroxybenzamido)7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxyphenol 10a
The crude product was purified by column chromatography utilizing ethyl acetate: hexane (3:1) to offered pale yellow precipitate. Yield 61%. Mp 102-104 °C. FTIR (KBr) υmax 3610 (OH phenol), 3345 (NH), 3060 (CHAr), 2990, 2879 (CHaliphatic), 1669 (C=O), 1625 (C=N), 1597, 1484 (C=C), 1342 (C-N), 1267 (Ar-O-C) cm-1. 1H-NMR (400MHz, DMSO-d6) δ; 3.85 (6H, s, 2× OCH3), 4.40 (2H, s, H-9), 4.68 (2H, s, CH2OCH2), 4.75 (2H, s, CH2OCH2), 6.62 (2H, s, H-3), 7.18 (2H,d, J 8.24, H-14), 7.69 (2H, d, J 8.2, H-13), 9.14 (1H, bs, OH). 9.87 (1H, bs, OH), 11.57 (1H, bs, NH). 13C-NMR (100 MHz, DMSO-d6) δ; 37.43 (1C, C-9), 58.03 (2C, 2×OCH3), 62.14 (1C, CH2OCH2), 70.55 (1C, CH2OCH2), 110.24 (2C, C-3), 119.72 (2C, C-13), 125.05 (1C-C-12), 129.81 (2C, C-14), 132.78 (1C, C-4), 139.29 (1C, C-1), 150.82 (2C, C-2), 151.84 (1C, C-15), 156.19 (1C, C=N), 157.63 (1C, C=N), 158.15 (1C, C=N). 173.46 (1C, C=O) HREIMs m/z = 471.1208 [M·+] (calc. for C21H21N5O6S, 471.1213).
4-(((6-(4-methylbenzamido)7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxyphenol 10b
The crude product was purified by column chromatography utilizing ethyl acetate: hexane (3:2) to offered pale yellow precipitate. Yield 64%. Mp 89-91 °C. FTIR (KBr) υmax 3604 (OH phenol), 3338 (NH), 3051 (CHAr), 2996, 2873 (CHaliphatic), 1671(C=O), 1631 (C=N), 1595, 1485 (C=C), 1343 (C-N), 1255 (Ar-O-C) cm-1. 1H-NMR (400MHz, DMSO-d6) δ; 2.29 (3H, CH3), 3.83(6H, s, 2× OCH3), 4.38 (2H, s, H-9), 4.76 (2H, s, CH2OCH2), 4.77 (2H, s, CH2OCH2), 6.59 (2H, s, H-3), 7.31 (2H, d, J 8.22,H-14), 7.60 (2H, d, J 8.4, H-13), 9.35 (1H, bs, OH), 11.39 (1H, bs, NH). 13C-NMR (100 MHz, DMSO-d6) δ; 21.24 (1C, CH3), 38.81 (1C, C-9), 57.74 (2C, 2×OCH3), 61.87 (1C, CH2OCH2), 71.03 (1C, CH2OCH2), 110.24 (2C, C-3), 126.55 (1C, C-12), 128.78 (2C, C-14), 130.01 (2C, C-13), 133.15 (1C, C-4), 139.29,. (1C, C-1), 140.80 (1C, C-15), 151.02 (2C, C-2), 157.16 (1C, C=N), 157.85 (1C, C=N), 158.92 (1C, C=N). 1732.29 (1C, C=O) HREIMs m/z = 469.1417 [M·+] (calc. for C22H23N5O5S, 469.1420).
4-(((6-(4-chlorobenzamido)7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxyphenol 10c
The crude product was purified by column chromatography utilizing ethyl acetate: hexane (3:2) to afford off white precipitate. Yield 68%. Mp 120-122 °C. FTIR (KBr) υmax 3557 (OH phenol), 3320 (NH), 3034 (CHAr), 2926, 2863 (CHaliphatic), 1668 (C=O), 1627 (C=N), 1598, 1480 (C=C), 1322 (C-N), 1247 (Ar-O-C) cm-1. 1H-NMR (400MHz, DMSO-d6) δ; 3.85 (6H, s, 2× OCH3), 4.43 (2H, s, H-9), 4.75 (2H, s, CH2OCH2), 4.79 2H, s, CH2OCH2), 6.61 (2H, s, H-3), 7.42 (2H, d, J 8.18, H-14), 7.76 (2H, d, J 8.20, H-13), 9.52 (1H, bs, OH), 11.21 (1H, bs, NH). 13C-NMR (100 MHz, DMSO-d6) δ; 39.05 (1C, C-9), 56.98 (2C, 2×OCH3), 62.37 (1C, CH2OCH2), 70.93 (1C, CH2OCH ), 110.13 (2C, C-3), 127.19 (1C, C-12), 128.65 (2C, C-14), 130.42 (2C, C-13), 133.15 (1C, C-4), 137.90 (1C, C15), 138.21 (1C, C-1), 151.44 (2C, C-2), 158.06 (1C, C=N), 158.25 (1C, C=N), 158.99 (1C, C=N). 172.71 (1C, C=O). HREIMs m/z = 489.0870 [M·+] (calc. for C21H20ClN5O5S, 489.0874).
Antioxidant
The DPPH assay was carried out as reported by Gerhauser et al. 24and the FRAP assay was carried out according to the Benzie and Strain 25 method as described in previous publications26,27. 2,6-dimethoxyphenol (2,6-DMP), BHT and ascorbic acid were used as standard references.
Results and Discussion
Chemistry
Newly compound 3 was synthesized from reaction equal equivalent of (3,5-dimethoxy-4-((trimethylsilyl)oxy)phenyl)methanol (1) with ethyl bromoacetate (2). Hydrolysis compound 3 in 50% acetic acid afforded compound (4) which is converted to their corresponding acid hydrazide (5). The acid hydrazide cyclized to their corresponding new 1,2,4-triazole-5-thione (6) as depicted in Scheme 1. Alkylation of compound 6 with chloroacetonitrile in the presence of potassium carbonate in acetone at room temperature afforded compound (7). Alkylation this compound temperature e.g up to 50°C will permit alkylation at phenolic hydroxyl group.
 |
Scheme 1: Synthetic route of synthesis compound 3-8 besides to 9a-c and 10a-c |
Refluxing compound (7) in acetic acid afforded corresponding fused 1,24-triazole-thiadiazine-6-amine ring (8). Compound 8 react with aryl isothiocyanate to afford the corresponding thiourea derivatives (9a-c), while it react with aryl acid chloride afford corresponding amide derivatives (10a-c). The para substituent and some physical properties were tabulated in Table 1. The structures of all synthesized compounds were confirmed from their FTIR, 1H NMR, 13C NMR besides to HREIMs spectra. The FTIR of compound 3 exhibited band at 1725 cm-1 attributed to carbonyl group which also appeared in 13C NMR spectrum at 171.04. The 1H NMR spectrum of this compound displayed new two singlet peak for CH2OCH2 as well the ethoxy group of ester as triplet for CH3 and quartet for CO2CH2. The FTIR of compounds 4-7 were convenient with proposed structure as well the 1H NMR and 13C NMR. Furthermore, the HREIMs spectra of these compounds were compatible with calculated mass. The FTIR spectrum of compound 8 was exhibited disappearance of the CN band at 2249 cm-1. Moreover it shows the band of OH at 3542 cm-1, while the band of NH2 located at3343 and 3219 cm-1 . The band of C=N was appeared at 1618 cm–1. The 1H NMR of this compound showed the two protons of H-9 at 4.41ppm and the two protons of NH2 at 7.34 as broad singlet peak besides to all expected protons were appeared at their expected regions . The 13C NMR also showed disappearance carbon signal of CN group as well it exhibited new peak at 37.57 ppm attributed to C-9 .three carbons signals at were located at 154.48, 157.16 and 157.70 ppm respectively attributed to three C=N for fused heterocyclic. The FTIR spectra of compounds 9a-c displayed new band at 3230-3235 cm-1 attributed to NH of thiourea part as well the band of C=S was located at 1247-1257 cm-1 besides to the popular band in their structure such as OH, CH aromatic & aliphatic and C=N. The 1HNMR spectra showed the new two doublet peaks each on with integration for two protons belonging to arylthiourea part, moreover the 1HNMR displayed peak at 2.21ppm for three protons of para CH3 of compound 9b and peak at 3.84 ppm for nine protons for three set of OCH3 for compound 9c. Furthermore, two broad singlets were located at 8.84-8.86 and 8.90-8.93 ppm attributed to two NH of thiourea part. 13C NMR spectra of these compounds were exhibited four new carbons attributed to arylthiourea part and interesting peak for C=S was located at 181.09-181.92 ppm besides to the expected carbons were located in their expected region. The accurate mass spectra (HREIMs) were in agreement with the structure and the molecular formula for synthesized compound 9a-c (Table 1).
Table 1: Substituent group and some physical properties of synthesized compounds
|
No. |
X
|
Yield % |
Mp C |
Molecular Formula |
HREIMs Found |
HREIMs Calc. |
|
8 |
– |
59 |
114-116 |
C14H17N5O4S |
351.0995 |
351.1001 |
|
9a |
Cl |
83 |
167-169 |
C21H21ClN6O4S2 |
520.0752 |
520.0754 |
|
9b |
CH3 |
85 |
134-136 |
C22H24N6O4S2 |
500.1294 |
50.1300 |
|
9c |
OCH3 |
78 |
128-130 |
C22H24N6O5S2 |
516.1247 |
516.1250 |
|
10a |
OH |
61 |
102-104 |
C21H21N5O6S |
471.1208 |
471.1213 |
|
10b |
CH3 |
64 |
89-91 |
C22H23N5O5S |
469.1417 |
469.1420 |
|
10c |
Cl |
68 |
120-122 |
C22H20ClN5O5S |
489.0870 |
489.0874 |
The FTIR spectra of compounds 10a-c showed interested two band, first one at xxxxcm-1 of NH and the second one at 1668-1671 to C=O which are indicated to successfully formation of amide. The 1H NMR spectra exhibited the peak of NH amide at xxx ppm and new peaks for aromatic protons of aryl part of the amide as two doublets for four protons, each one integral for two protons. Furthermore, new broad singlet peak was located with compound 10a attributed to para OH at 9.87 ppm. The para CH3 of compound 10b was located at 2.29 ppm. The 13C NMR spectra of these compounds showed characteristic peak for carbonyl of amide at 172.71-173.46 ppm. Furthermore, the spectra exhibited all carbons of aryl amide group at their expected regions. The HREMs spectra were compatible with the calculated molecular mass.
Antioxidant Activity
The antioxidant ability of newly synthesized compounds 6-8, 9a-c and 10a-c were evaluated with DPPH assay which prefer atom transfer mechanism (HAT)28. Furthermore, the antioxidant ability of these compounds were evaluated utilizing FRAP assay which is undergoes single electron transfer mechanism (SET)29 . The DPPH inhibition % and the IC50 value for these compounds tabulated in Table 2. Compound 6 exhibited antioxidant ability higher than BHT and less than ascorbic acid. And their IC50 value was less BHT. The antioxidant of compound 7 (alkyl derivative of compound 6) showed eminent deficiency in antioxidant ability. This deficiency could be point to fade the thioamide group which were reported as free radical scavengers.30 Thioamide is considered as a part of thiourea system. Compound 8 showed DPPH inhibition slightly less than BHT as well their IC50 is higher than BHT. The antioxidant capacity of compounds 9a-c showed significant antioxidant capacity. Compound 9b exhibited DPPH inhibitions higher than ascorbic acid, although their IC50 was higher than ascorbic acid. Compound 9c exhibited antioxidant less than ascorbic acid and 9a showed DPPH inhibition less than compound 9b and 9c. Increase the antioxidant ability of compounds 9a-c when compared to antioxidant ability of compound 8 could be attributed to thiourea system which is known as effective antioxidant31. Furthermore, the difference in DPPH results between 9a, 9b and 9c approve that the inductive effect of electron-donating groups (EDG) of substituent group at para position play vital role to enhance the antioxidant ability, while the as electron-withdrawing groups (EWG) demote the antioxidant ability. Compounds 10a-c exhibited moderated antioxidant ability. Their antioxidant was higher than compound 8, but less than 9a-c. Compound 10a showed higher antioxidant than 10b and 10c and that could due the existence of another phenolic hydroxyl group which can enhances the antioxidant properties.
Table 2: DPPH inhibition % and IC50 for synthesized compounds 6-8,9a-c and 10a-c
|
Compound No. |
x |
DPPH Inhibition % ± SD a |
IC50 ±SEM b (100µg/mL) |
|
6 |
– |
69.07 ±0.034 |
81.77 ±0.044 |
|
7 |
– |
58.26 ±0.058 |
> 100 |
|
8 |
– |
63.91 ±0.011 |
> 100 |
|
9a |
Cl |
79.71 ±0.024 |
30.8±0.01 |
|
9b |
CH3 |
92.81 ±0.035 |
56.43±0.016 |
|
9c |
OCH3 |
83.67 ±0.021 |
61.18±0.035 |
|
10a |
OH |
75.37 ±0.028 |
65.4±0.071 |
|
10b |
CH3 |
70.51 ±0.020 |
> 100 |
|
10c |
Cl |
68.09 ±0.047 |
> 100 |
|
2,6- DMP |
– |
42.16 ±0.078 |
> 100 |
|
BHT |
– |
66.23 ±0.025 |
78.64±0.015 |
|
Ascorbic acid |
– |
89.34 ±0.025 |
21.50±0.020 |
a Standard deviation (SD) value in FRAP was between 0.01–0.2; b SEM standard error of mean and IC50: 50% effective concentration.
The FRAP value of these compounds (Figure 1) were harmonious with the DPPH results. These results indicate that these compounds capable to undergoes with HAT and SET mechanisms.
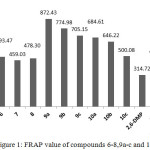 |
Figure 1: FRAP value of compounds 6-8,9a-c and 10a-c Click here to View figure |
Conclusions
4-amino-3-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)-1H-1,2,4-triazole-5(4H)-thione 6 successfully converted to their corresponding thioacetonitrile derivatives 7 which cyclized to obtain 4-(((6-amino-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)methoxy)methyl)-2,6-dimethoxyphenol 8 two series were synthesized from compound 8. First one was as the corresponding thiourea derivative 9a-c. The second one was as the corresponding amid derivatives 10a-c. All compounds were characterized successfully and screened their antioxidant ability. Compound 9a showed significant antioxidant ability, moreover 9a-c exhibited antioxidant higher than 10a-c and that could be attributed to existent of thiourea part which enhances the antioxidant ability.
Acknowledgements
The authors would like acknowledge University of Baghdad for their supporting and funding of this research. As well we would like to present our thanks to University of Malaya for their cooperation.
References
- Shacter, E. Drug Metabolism Reviews 2000, 32, 307.
CrossRef - Chatterjee, R.; Bandyopadhyay, U.; Mazumdar, A.; Banerjee, R. K. Biochemical Pharmacology 1996, 52, 1169.
CrossRef - Sangeetha, P.; Das, U. N.; Koratkar, R.; Suryaprabha, P. Free Radical Biology and Medicine 1990, 8, 15.
CrossRef - Kar, S.; Subbaram, S.; Carrico, P. M.; Melendez, J. A. Respir Physiol Neurobiol. 2010, 174, 299.
CrossRef - Lin CC, H. P. Phytotherapy Research 2000, 14, 489.
CrossRef - Raied M. Shakir; Azhar Ariffin; Mahmood Ameen Abdulla Molecules 2014, 19, 3436.
- Hung, C.-Y.; Yen, G.-C. J. Agric. Food Chem. 2002, 50, 2993.
CrossRef - Ju-Mi Jeong , S.-K. K., In-Hwa Lee, Ji-Yoon Lee, Hyuk Jung, and Cheol-Hee Choi J Pharm Pharmaceut Sci 2007, 10, 537.
- Edge, R.; McGarvey, D. J.; Truscott, T. G. Journal of Photochemistry and Photobiology B: Biology 1997, 41, 189.
CrossRef - G.R. Bakhshandeh, M. T. K. iranian journal of polymer science and technology 1992, 1, 62.
- Cui, P.; Li, X.; Zhu, M.; Wang, B.; Liu, J.; Chen, H. European Journal of Medicinal Chemistry 2017, 127, 159.
CrossRef - Kamath, P. R.; Sunil, D.; Das, S.; Ajees, A. A.; Rao, B. S. S. Chemico-Biological Interactions.
- Zaki, M.; Allouchi, H.; El Bouakher, A.; Duverger, E.; El Hakmaoui, A.; Daniellou, R.; Guillaumet, G.; Akssira, M. Tetrahedron Letters 2016, 57, 2591
CrossRef - Liu, X.-H.; Zhai, Z.-W.; Xu, X.-Y.; Yang, M.-Y.; Sun, Z.-H.; Weng, J.-Q.; Tan, C.-X.; Chen, J. Bioorganic & Medicinal Chemistry Letters 2015, 25, 5524.
CrossRef - Ruiz-Alcaraz, A. J.; Tristán-Manzano, M.; Guirado, A.; Gálvez, J.; Martínez-Esparza, M.; García-Peñarrubia, P. European Journal of Pharmaceutical Sciences 2017, 99, 292.
CrossRef - Chaturvedi, B.; Tiwari, N.; Nizamuddin Agricultural and Biological Chemistry 1988, 52, 1229.
- Wang, L.; Tian, Y.; Chen, W.; Liu, H.; Zhan, P.; Li, D.; Liu, H.; De Clercq, E.; Pannecouque, C.; Liu, X. European Journal of Medicinal Chemistry 2014, 85, 293.
CrossRef - Sompalle, R.; Arunachalam, P.; Roopan, S. M. Journal of Molecular Liquids 2016, 224, Part B, 1348.
- Radwan, R. R.; Zaher, N. H.; El-Gazzar, M. G. Chemico-Biological Interactions 2017, 274, 68.
CrossRef - LaPorte, M. G.; Wang, Z.; Colombo, R.; Garzan, A.; Peshkov, V. A.; Liang, M.; Johnston, P. A.; Schurdak, M. E.; Sen, M.; Camarco, D. P.; Hua, Y.; Pollock, N. I.; Lazo, J. S.; Grandis, J. R.; Wipf, P.; Huryn, D. M. Bioorganic & Medicinal Chemistry Letters 2016, 26, 3581.
CrossRef - Sumangala, V.; Poojary, B.; Chidananda, N.; Arulmoli, T.; Shenoy, S. European Journal of Medicinal Chemistry 2012, 54, 59.
CrossRef - Huihui Ti; Qing Li; Ruifen Zhang; Mingwei Zhang; Yuanyuan Deng; Zhencheng Wei; Jianwei Chi; Yan Zhang Food Chemistry 2014, 159 166.
- Fausta Natella, M. N., Maurizio Di Felice, and Cristina Scaccini*; Free Radical Research Group, N. I. o. N., Roma, Italy J. Agric. Food Chem. 1999, 47, 1453.
- Gerhäuser, C.; Klimo, K.; Heiss, E.; Neumann, I.; Gamal-Eldeen, A.; Knauft, J.; Liu, G.-Y.; Sitthimonchai, S.; Frank, N. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2003, 523-524, 163.
CrossRef - Benzie, I. F.; Strain, J. J. Anal. Biochem. 1996, 239, 70.
CrossRef - Shakir, R. M. Oriental Journal Of Chemistry 2016, 32, 2611.
- Saoud S. A; Ali K. F; Shakir R. M Orient J Chem 2017, 33, 1781.
- E. A. Braude ; A. G. Brook; Linstead, R. P. J. Chem. Soc 1954, 3574.
CrossRef - Brand-Williams, W.; Cuvelier, M. E.; Berset, C. LWT – Food Science and Technology 1995, 28, 25.
CrossRef - Velkov, Z.; Balabanova, E.; Tadjer, A. Journal of Molecular Structure: Theochem 2007, 821, 133.
CrossRef - Ozdem S; Alicigüzel Y; Ozdem, S. S.; Karayalçin, U. Pharmacology 2000, 61, 31.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









