Silver Colloid and Silver Mirror Complex as Surface Enhanced Raman Substrate for Volatile Organic Compounds Analysis
Sarunrat Injai-Uea1, Natphichon Budtri2 and Weerasak Lertsiriyothin1
1School of Agricultural Engineering, Institute of Engineering, Suranaree University of Technology, 111 University Avenue, Muang District, Nakhon Ratchasima, 30000, Thailand.
2School of Mechanical and Process System Engineering, Institute of Engineering, Suranaree University of Technology, 111 University Avenue, Muang District, Nakhon Ratchasima, 30000, Thailand.
Corresponding Author E-mail: Sarunrat.i@my.nsru.ac.th
DOI : http://dx.doi.org/10.13005/ojc/330540
This research focuses on the development of Raman spectroscopy for determination of the low volatile concentration as toluene volatile and 2-Acetyl-2-thiazoline (2AT) volatile. The studies were divided into two techniques. The research was to develop surface enhanced Raman substrates (SERS) enhanced the surface using silver metal for enhancement. In this part, there were two different types of the SERS suitable for FT-Raman. The first technique was silver colloid. This technique could build nanosilver particles, of which average size was 35.5 – 140.3 nm. The detection limits were found to be at 4000 ppm in 2AT solution. The second techniques were the silver mirror and freezing technique. The detection limits for 2AT solution 0.2 ml in vial were found to be at 4000 and 1000 ppm in the silver mirror. Results of the enhanced Raman spectra of volatile organic compounds (VOCs) contained in a sample showed that the concentration of 2AT was existed at higher level than that of toluene. Therefore, the silver mirror type of SERS together with the FT-Raman could be used for determining 2AT in low concentration.
KEYWORDS:SERS; Raman Spectroscopy; Silver mirror; Silver colloid; VOCs
Download this article as:| Copy the following to cite this article: Injai-Uea S, Budtri N, Lertsiriyothin W. Silver Colloid and Silver Mirror Complex as Surface Enhanced Raman Substrate for Volatile Organic Compounds Analysis. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Injai-Uea S, Budtri N, Lertsiriyothin W. Silver Colloid and Silver Mirror Complex as Surface Enhanced Raman Substrate for Volatile Organic Compounds Analysis. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=38679 |
Introduction
Raman and Infrared spectroscopy are analytical techniques for studying and inspecting a spin or vibrate transition of functional group of molecular structures in solid, liquid, and gas states. The both techniques are different in processing. The electrostatic irradiation with chemical molecule Infrared spectroscopy technique is the infrared irradiation absorption processing as infrared light interacting with a molecule. It can be analyzed by measuring absorption, emission, and reflection. Organic and inorganic chemistry are measured by this technique to determine functional groups in molecules. Raman spectroscopy technique is the technique of C.V. Raman who was honored with its inventor along with K.S. Krishnan1. It is a scattering technique for detecting quantitative and qualitative samples. The superlow concentration of sample is a problem to be analyzed with the Raman spectrum. The surface enhanced Raman scattering (SERS) is one of the techniques for increasing Raman spectrograph.
SERS is the technique enhancing limited ability of the Raman spectroscopy for the determination of the superlow concentration in ppm level2. They have reported that the limited importance of this technique is the low concentration of chemical volatile or gas. Zhang3 reported that the determination of low volume nitro toluene was detected by FT-Raman. The samples were in closed system because they could control volatile dispersion in the system. This method could not detect low volume in environment. Mosier-Boss4 has studied about a transient electrochemical surface-enhanced Raman spectroscopy (TEC-SERS) system. It is a continuous determined system so the system requires high volume of samples. Silver is a one of SER substrates to be air stable material5. Silver colloid was used be a SER substrate because it can give reproducible and sensitive analysis6.The SERS is not used for volatile measurement in full potential. Furthermore, the SERS technique is especially applied in food flavor which is the important thing for food quality and consumer acceptance. It can indicate composition characteristics, deterioration, and contamination such as changed flavor made by bacterial fermentation. The synthetic flavor is added to food products for making high quality food. Furthermore, SERS can also indicate low volume of 2-Acetry-1-pyrroline in Jasmine rice (Kao Hom Mali)7-11. It is an aromatic compound having the same flavor as that in Pandanus.leaf. The volatile compound is also used in agricultural product of Thailand such as KaoHom Mali and KaoHomPrathumtani. They have identical appearance and same volatile compounds as 2-Acetyl-2-thiazoline. 2-Acetyl-2-thiazoline is also important aroma compound identified in cooked rice, beef flavor, and another food flavor7, 12-13. Furthermore, Toluene is the index of contamination in food industry. The SERS substrates also match each chemical form such as colloidal metal solution, metal coating on surface, and chemical reaction with metal.
This research was to study about SERS substrates of silver metal enhancing the Raman spectrograph and the development of SERS technique for determining the low volume and low concentration of samples. The SERS substrates were prepared from colloidal silver solution and silver mirror on glass slide. Toluene and 2AT were used to be samples then the SERS were determined by Raman spectroscopy.
Materials and Methods
Materials
Silver nitrate (AgNO3), Toluene (C7H8), 2-Acetyl-2-thiazoline (C5H7NOS), Sodium hydroxide (NaOH), ammonia, and glucose (C6H12O6) were purchased from Sigma Aldrich. All water was deionized for experimental usage.
Preparation of SERS Substrates
Silver colloid was obtained according to citrate reduction method or Lee and Meisel method14. It was prepared by adding 90 mg of AgNO3 into 500 ml of distilled water at 45°C. The solution was mixed, boiled, and incessantly stirred on the hot plate. 10 ml of 1% (w/w) sodium citrate was added and continuously boiled for 1 h.The solution was refluxed while it was boiled. Silver colloid was slowly cooled in room temperature. The silver colloid particle was measured by particle analyzer.
Silver mirror was obtained according to Tollens method15. It was prepared by putting 1 ml of 1 mM AgNO3 into the tube and adding concentrated ammonia to the solution. A brown precipitate will form and concentrated ammonia was added until the solution became clear. After that, 0.3 M of NaOH solution was added to adjust pH (10.5±0.1). The solution was placed on the glass slide and then 1 ml of 0.1 mM glucose solution was added. The solution would turn to be yellow and brown then it became cloudy and dark before silver began to form on a glass slide. Silver mirror solution was reacted until the completed reaction. The glass slides were dried in room temperature.
Preparation of Volatile Samples
2AT and toluene were used to be volatile samples for SERS testing. 2AT solution were prepared in DI water (100, 500, 1000, and 4000 ppm), incubated at 60°C for 12 h, and liquid was dropped in nitrogen for 5 minutes before testing. 0.2 ml of toluene was prepared in vial, incubated at 60°C for 12 h, and dropped in liquid nitrogen for 5 minutes before testing silver mirror substrates. 0.5 ml of 2AT solution was added into 0.5 ml of silver colloid substrate solution in quartz cuvette and 0.5 ml of toluene solution was also added.
Raman Measurement
The SERS and Raman spectra signals were collected by a Fourier transform Raman spectrometer (Bruker, Ram II) using 1064 nm excited Nd:YAG laser and 64 scan times in room temperature. The spectral range between 4000 and 0 cm-1 was simultaneously used for recording at resolution 4 cm-1 of FT-Raman apparatus. Data of Raman spectra were analyzed by Opus program.
Results and Discussion
Characterization of Sers Substrate Development
The development of silver colloid was used to be SERS by citrate reduction method. The solution of silver colloid was in yellow-gray color. It has been kept at 4°C for 2 months. The silver particles were measured by particle analyzer. They were nanoparticles size range between 35.5-140.3 nm and diameter of 113 nm. The result was similar to Zhang16 who reported that the silver particle sizes with an average diameter of 13.8 nm, and 35-500 nm of silver colloid particle sizes reported by Kim17. Furthermore, Prucek15 and Lin18 reported a range of nanosphere sizes between 430 to 1500 nm.
The silver mirror was developed to be SERS by Tollens’s method15. The silver mirror surface was emerged in a yellow-gray color like silver colloid color. Figure 1 shows the surface roughness mirror. The result shows that the roughness of silver particles was not separated all over surface on glass slide. Similar to Bu19 who reported that the particle sizes were indicated to be nanoparticles size with Au and Ag.
 |
Figure 1: A silver mirror surface from Raman microscope. Click here to View figure |
Effect of SERS Substrates on Raman Scattering Signal
The chemical substance 2-Acetyl-2-thiazoline (2AT) and toluene in this research were volatile compounds. The concentration of 2-Acetyl-2-thiazoline was prepared from 100 to 1,000 ppm and it was tested by FT-Raman spectroscopy. Figure 2 shows the Raman spectrum of 2AT which was used the silver colloid for the media to increase the signal of Raman spectrum. The results showed that Raman peak spectrum (Figure 2a) indicated 2AT vapor at wave number 1437 cm-1 and the peak shows the weak vibrational band and this band corresponds to C-N stretching motion. The spectrum of Raman peaks at 612 cm-1, 1595 cm-1 and 1701 cm-1 show the strong vibrational bands. Their rendition are ring stretching, C-S stretching, C=C stretching, and C=H stretching, respectively. A very strong vibrational band is observed at 2913 cm-1. The band can be assigned to CH3 stretching vibration. The spectrum signals did not indicate the 2AT peak between 3000-3500 cm-1 but the signal was shifted to 3222 cm-1 of wave number. They might also be affected by laser heat. 0.5 ml of silver colloid and 0.5 ml of 2-Acetyl-2-thiazoline were mixed together. The peak spectra of 2AT did not clearly appear at 100, 500, and 1000 ppm. The concentration of 4000 ppm of 2AT did not show the peak signal at 612 cm-1, 1437 cm-1, 1595 cm-1, 1701 cm-1 and 2913 cm-1 but the signals were shifted as shown in Figure 3. Because, the colloidal silver solution was precipitated and accumulated for 3-4 seconds. Eventually, the sample was not continuously homogenous to Raman spectrum measurement and similar to the report of Munro14 who found the colloidal silver nanocrystal structure in low dispersion, precipitation, and accumulation. Raman spectrum shift was found with a minor bathochromic shift to high wave length for 2, 10, 15, and 90 min such as the initial measurement at the peak of 1390 cm-1. The spectrum shift was changed to the peak of 1395, 1400, and 1400 cm-1 for 10, 15, and 90 min, respectively. The colloidal silver technique was suitable for stirring in suspension. Zhang3 reported the measurement of 2,4,6 trinitrotoluene (TNT) using confocal microprobe Raman system at laser source 532 nm by adding air bubbles to flow through polymer tube into colloidal silver solution. The result showed that the minimum concentration for detection was a 10 µg/l. Lin18 reported that the optimum SER signal was obtained at an excitation wave length of 532 nm with a nanosphere size of 1000 nm.
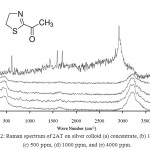 |
Figure 2: Raman spectrum of 2AT at concentration (a) concentrate, (b) 100 ppm, (c) 500 ppm, (d) 1000 ppm, and (e) 4000 ppm. Click here to View figure |
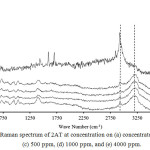 |
Figure 3: Raman spectrum of 2AT at concentration (a) concentrate, (b) 100 ppm, (c) 500 ppm, (d) 1000 ppm, and (e) 4000 ppm. Click here to View figure |
Silver mirror technique was used in this research measured by using toluene and 2AT. Toluene was absorbed by silver mirror glass at temperature 60 °C and was measured by FT-Raman spectroscopy. Toluene peak spectrum on silver mirror glass was not shown as seen in Figure 4 because molecules of toluene in gas state had low opportunity for collisions and binds within silver mirror. The experimentation was closed system and gas molecularity was also in low mobilization. The technique for measurement was immediate freezing technique binding the molecule of toluene within the silver mirror. The procedures were firstly preparing silver mirror glass and toluene which was contained in vial, and then immersing them to liquid nitrogen before measuring. The results showed that Raman peak spectrum indicated toluene vapor at 783 cm-1, 1030 cm-1, 1211 cm-1, 1611 cm-1, and 3056 cm-1. This technique increased by 5 times of spectrum signals compared with toluene Raman spectrum of toluene within silver mirror without immersing liquid nitrogen. Raman spectrum of 2AT in gas state indicating the measurement of concentration between 612 cm-1 and 2913 cm-1 with the minimum concentration of 2AT at1000 ppm was from 100, 500, 1000, and 4000 ppm as shown in Figure 5.
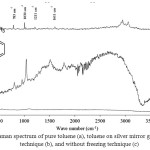 |
Figure 4: Raman spectrum of pure toluene (a), toluene on silver mirror glass by freezing technique (b), and without freezing technique (c) Click here to View figure |
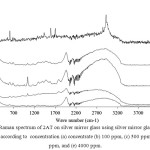 |
Figure 5: Raman spectrum of 2AT on silver mirror glass using silver mirror glass freezing technique according to concentration (a) concentrate (b) 100 ppm, (c) 500 ppm, (d) 1000 ppm, and (e) 4000 ppm. Click here to View figure |
Table 1: Comparison of volatile measurement technique using Raman spectroscopy.
|
No |
Type |
Laser source (nm) |
Samples |
Min. concentration |
Technical |
System |
Reference |
|
1 |
FT-Raman |
1064 |
2-Acetyl-2-thiazoline (2AT) |
1000 ppm |
SERS Silver mirror |
Closed |
This Research |
|
2 |
Dispersive |
785 |
Benzene, Toluene, Ethylbenzene, and Xylenes |
– |
Thiol-coated onthermoelectric cooler |
Continuous |
[4] |
|
3 |
Confocal microprobe Raman (Dispersive) |
532 |
2,4,6 Trinitrotoluene (TNT) |
10 µg/L |
Silver colloid |
Continuous |
[3] |
|
4 |
Raman microscope |
633 |
Acetone and Ethanol |
0.8% and 0.4% |
Nano-pillar substrate |
– |
[18] |
Wong20 reported that nano-pillar substrate development using Raman microscope Laser source at 633 nm could detect a minimum of 0.4% of ethanol and 0.8% of acetone by sample flow through system. This technique was reported by Zhang3 that it showed the determination of volatile organic compounds in low concentration (ppm) as shown in table 1. Raman spectroscopy was remarkable for determining the chemical volatile in low concentration. It could specify chemical structures and chemical compounds. This research found that the SERS developing silver mirror glass with freezing were our techniques. They could support the FT-Raman laser source at 1064 nm as low activated energy of Raman spectroscopy. They could also detect and measure the low volatile compounds (0.2 ml) in closed system. The SERS were used in this experiment for their practical production at a low price.
Conclusions
In this study, silver colloid and silver mirror were used to be a SERS for increasing the activated energy of Raman spectroscopy. Silver colloids were prepared in solution as nanoparticles size range between of 35.5-140.3 nm and diameter of 113 nm. The increasing Raman spectrum of 2AT in silver colloids was not changed when compared with Raman spectrum of 2AT. Silver mirror was prepared by Tollens method on glass slide and the determination of toluene and 2AT. They were both used for an immediate freezing technique and unfreezing. Raman spectrum increased by almost 5 times of 2AT. They could measure the volatile compounds (0.2 ml) in low volume and a closed system.
Acknowledgments
This work was supported by National Research Council of Thailand (NRCT), Office of the Higher Education Commission (OHEC), and Suranaree University of Technology (Nakhon Ratchasima, Thailand).
References
- Raman, C. V.; Krishnan, K. S. Nature. 1928, 121, 501–502.
CrossRef - Deschaines, T. O.; Wieboldt, D. Practical Applications of Surface-Enhanced Raman Scattering (SERS).Technical Note: 51874, Thermo Fisher Scientific, Madison, WI, USA 2010.
- Zhang, C.; Wang, K.; Han, D.; Pang, Q. Spectro Acta Part A: Molecur and Biomolecur Spectro. 2014, 122, 387 -319.
CrossRef - Sharma, B.; Frontiera, R. R.; Henry, A. I.; Ringe, E.; Van Duyne, R. P. Materialstoday. 2012, 15, 16-25.
CrossRef - Munro, C. H.; Smith, W. E.; Garner, M.; Clarkson, J.; White, P. C. Langmuir. 1995, 11, 3712-3720.
CrossRef - Mosier-Boss, P. A.; Lieberman, S. H. Anal. ChimicaActa. 2003, 488, 15–23.
- Buttery, R. G.; Ling, L. C.; Juliano, B. O.; Turnbaugh, J. G. J. Agric. Food. Chem. 1983, 31, 823-826.
CrossRef - Jezussek, M.; Juliano, B. O.; Schieberle, P. J. Agric. Food. Chem. 2002, 50, 1101-1105.
CrossRef - Laksanalamai, V.; Ilangantileke, S. Cere. Chem. 1993, 70, 381 – 384.
- Paule, C. M.; Power, J. J. food sci. 1989, 54, 343 – 347.
CrossRef - Mahattanatawee, K.; Rouseff, R. L. Food Chem. 2014, 154, 1-6.
CrossRef - Fang, M. C.; Cadwallader, K. R. J. Agric. Food Chem. 2014, 62, 8808-8813.
CrossRef - Plancken, A. J.; Tonsbeek, C. H. T.; Copier, H. J. Agric. Food Chem. 1971, 19, 1014–1016.
CrossRef - Munro, C.H.; Smith, W.E.; Garner, M.; Clarkson, J.; White, P.C. Langmuir. 1995, 11, 3712-3720.
CrossRef - Prucek, R.; Ranc, V. C.; Balzerová, O.; Panácêk, A.; Zbořil, R.; Kvítek, L. Materi. Res. Bul. 2014, 50, 63–67.
CrossRef - Zhang, Z.; Wu, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Materi. Sci. Eng. C. 2016, 69, 462-469.
CrossRef - Kim, K.; Jang, H. J.; Shin, K. S. Royal. Soci. Chem. 2008, 134, 308 – 313.
- Lin, W.C.; Liao, L. S.; Chen, Y. H.; Chang, H.C.; Tsai, D. P.; Chiang, H. P. Plasmonics. 2011, 6, 201–206.
CrossRef - Bu, Y.; Park, S. J.; Lee, S. W. Current. Appl. Phys. 2014, 14, 784-789.
CrossRef - Wong, C. L.; Dinish, U. S.; Schmidt, M. S.; Olivo, M. AnalyticaChimicaActa. 2014, 844, 54-60.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









