Removal of 2-Chlorophenol using Rice Husk Activated Carbon Prepared by ZnCl2/H3PO4 Activation
Allwar Allwar*, and Syamsurizal
Department of Chemistry, Faculty Mathematics and Natural Sciences Islamic University of Indonesia, Yogyakarta, 55584, Indonesia.
Corresponding Author E-mail: allwar@uii.ac.id
DOI : http://dx.doi.org/10.13005/ojc/330531
The rice husk, agricultural solid waste was successfully converted into activated carbon using mixture of chemical agents ZnCl2/H3PO4. Their properties including porous structures, surface functional groups and morphology structures were carefully studied. Applications of rice husk activated carbons were applied for removal of 2-chlorophenol in aqueous solution under different conditions. The nitrogen isotherm exhibits Type IV, and the presence of hysteresis loop clearly shows the predominantly mesoporous characteristics. The BET and Langmuir surface areas are 144.6 and 212.5 m2g-1, respectively. The equilibrium adsorptions for both Langmuir and Freundlich isotherms show the best a regression coefficient closed to unity. The values of correlation coefficient R2 = 0.9976 represented the satisfactory pseudo-second-order model. The results show that rice husk activated carbon was effectively used as adsorbent for removal of 2-chlorophenol.
KEYWORDS:Rice husk; Activated carbon; 2-chlorophenol; ZnCl2/H3PO4
Download this article as:| Copy the following to cite this article: Allwar A, Syamsurizal S. Removal of 2-Chlorophenol using Rice Husk Activated Carbon Prepared by ZnCl2/H3PO4 Activation. Orient J Chem 2017;35(5). |
| Copy the following to cite this URL: Allwar A, Syamsurizal S. Removal of 2-Chlorophenol using Rice Husk Activated Carbon Prepared by ZnCl2/H3PO4 Activation. Orient J Chem 2017;35(5). Available from: http://www.orientjchem.org/?p=39313 |
Introduction
Activated carbon is a solid material with relatively small particle sizes: powder or granular form having excellent characteristics as adsorbent. The characteristics can be separated in physical and chemical properties and make them suitable for application in many catalytic processes. Activated carbon is highly porous structures and sufficiently surface functional group while its activity is studied in the adsorption process involving as adsorbent, catalyst and catalyst support in chemical reaction. It is capable to absorb a wide variety of pollutants including organic pollutant, dyes and heavy metals [1]. Porous structures of adsorbent must be excellent characteristics including high surface area, pore volume and pore size distribution. These properties are essentially performance indicators as adsorbent for removal of contaminant from aqueous solution. The highly porous structures have a strong interaction between adsorbate molecules and active site of carbon. Graphene structures of activated carbon contain with electron-rich-regions and surface functional groups with play important roles for determination of removal of pollutant. Two assumptions of mechanism can be describe that the π electron on the graphene layers may interact with π- π electrons of adsorbate, and basic surface functional groups such as carbonyl on surface may bond the aromatic ring of phenolic compound through donor acceptor interaction [2, 3].
2-chlorophenol as a phenolic organic aromatic compound can be obtained as water pollutants and deliberated as major toxic pollutants event in low concentration as low as 0.1 ppm. This chemical is used as intermediate process in the synthesis of resin, plastics, dye, pesticides, insecticide and many others [4, 5]. The polluted waste drains into natural water, canal, river, etc. representing a serious problem and affecting not only human life but also flora and fauna as well [6, 7]. In the last few years, a great attention had been focused on the detection and removal of 2-chlorophenol and phenolic derivatives due to uncontrolled discharging of industries effluents and drinking water disinfection. Various treatment technologies are used for removal of the pollutants before discharging into the water body including adsorption, ion-exchange, chemical reaction, reverse osmosis, electrodialysis and filtration [8-10].
Previous works have been reported that activated carbon can be obtained from various raw materials possessing highly carbonaceous compounds such as coconut shell, kernel, bamboo, rice husk, tire, etc . Rice husk is an agricultural solid waste from farmer which is easily obtained around milling process and inexpensive material. The compositions of rice husk have been studied and being investigated for preparation of activated carbon as adsorbent for removal of organic pollutants. Improving the quality of porous structures and adsorption capacity has been intensively studied. Development of technologies for preparation of high porous activated carbon is very important to obtained maximum process in removal of contaminates [11-13].
In the present work, activated carbon was prepared from waste of rice husk and used as an adsorbent for removal of 2-chlorophenol. Preparation activated carbon was carried out with hydrothermal method using mixture chemical agents ZnCl2/H3PO4. Characteristics of activated carbon were applied by Surface Area analyzers Quanthachrome , FT-IR spectroscopy and SEM-EDX. Adsorption capacity was determined at various parameters including: pH, contact times, concentration and adsorbent dosage. The amount of 2-chlorophenol uptakes was evaluated by UV Visible Spectrophotometer at wavelength of 274 nm. Adsorption isotherms data were used for studying the Langmuir and Freundlich adsorption isotherm including kinetic models.
Materials and Methods
Materials
Rice husk can be easily obtained at open area around milling process or rice field. It was washed thoroughly with distilled water to remove impurities attacked on the rice husk and dried in an oven at 120 °C overnight. The chemicals involving zinc chloride (ZnCl2), phosphorous acid (H3PO4), buffer solution, 2-chlorophenol are obtained from E Merck and used without further purification.
Preparation of Rice Husk Activated Carbon
About 200 g of rice husk activated carbon was prepared by pyrolysis proses at constant temperature 200°C for 2 h. After cooling down, the 150 g of biochar of rice husk was poured into 300 mL of ZnCl2/H3PO4 solution obtained from mixtures 10% ZnCl2 and 10% H3PO4. The Mixture was refluxed at 80oC for 24 h. Therefore, it was filtered and washed with distilled water for few times and neutralized until its pH 6 – 7. The dried sample was taken into close reactor and pyrolyzed in graphite furnace at 300oC for 2 h as a contact time. The dried production was rice husk activated carbon and kept in desiccators for further analysis.
Procedures of Adsorption of 2-chlorophenol
To study the advance adsorption process for 2-chlorophenol removal, some different parameters were applied during adsorption process including pH (pH= 4, 5, 7, 8), adsorbent dosage (0.5, 1.0, 1.5, 2.0 g), contact times (15, 30, 45, 60 min) and initial 2-chlorophenol concentration (50, 100, 150, 200 mg/L). The amount of 2-chlorophenol removal was calculated using UV-Vis spectrophotometer at wavelength of 274nm. Equilibrium adsorption isotherm was evaluated using Langmuir and Freundlich adsorption isotherm including its kinetic process [14]. The amount of 2-chlorophenol removed by the rice husk activated carbon was studied using batch technique in a shaker at room temperature. The adsorption capacity (qe) and the removal % (R, %) were evaluated using the following equation (1):
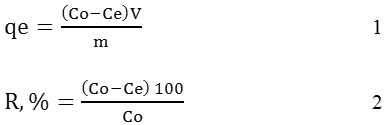
Where, Co and Ce are the initial and the equilibrium concentrations (mg/L) of phenol and 2-chlorophenol. The V is volume of the solution (L) and m is the mass of adsorbent (gr) [15] .
Results and Discussion
Surface area Analysis
Textural characteristics of activated carbon were carried out based on the nitrogen adsorption and desorption isotherms at 77 K. Fig. 1 shows the shape of nitrogen isothermal exhibited IV isotherms corresponding to the great development of mesoporous structures based on the IUPAC classification: micropores (8 to100 Å), mesopores (100–500 Å) and macropores (>500 Å) [16, 17]. The presence of hysteresis loop at P/Po >0.4 were clearly from multilayer condensation of physisorption associated with the filling and emptying of mesopores. Surface areas were evaluated at different relative pressure in which BET (Brunauer-Emmett-Teller) and Langmuir methods range from P/Po 0.05 to 3.0, and P/Po < 0.05, respectively. The results show that BET surface area is 144.6 m2g-1 corresponding with the multilayer, and Langmuir surface area is 212.5 m2g-1 corresponding with monolayer. Fig. 2 shows the BJH shape for the distribution of radius pores started from 16 to 200 Å. From the data presented in the Fig. 2, it can be seen that the rice husk activated carbon produced the predominantly mesoporous structures. Previous work reported that preparation of activated carbon using chemical activation with ZnCl2 have produced activated carbon with microporous characteristics, while with H3PO4 resulted mesoporous structure. Therefore, due to the effect of combination of ZnCl2 and H3PO4, the pores have shifted from microporous to mesoporous structures. The textural characteristics of rice husk activated carbon can be concluded that it was posted in Table 1.
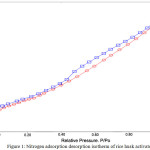 |
Figure 1: Nitrogen adsorption desorption isotherm of rice husk activated carbon. Click here to View figure |
 |
Figure 2: BJH shape of pore size distribution of rice husk activated carbon. Click here to View figure |
Table 1: Textural characteristic of rice husk activated carbon.
| Langmuir surface area (m2g-1) | BET surface area (m2g-1) | DR Micropore volume (ccg-1) | BJH Pore volume(ccg-1) | DA Pore radius(Å) | BJH Pore radius (Å) |
| 212.5 | 144.6 | 0.064 | 0.2 ccg-1 | 8.8 | 18.3 |
Functional groups of Activated Carbon
Investigation of the functional groups attached on the surface of activated carbon was carried out by FTIR. Fig. 3A shows FTIR spectra of biochar of rice husk that was obtained from pyrolysis process at 200oC. Fig. 3B shows FTIR spectra of rice husk activated carbon that was prepared by the mixtures of chemical agents ZnCl2/H3PO4. The effect of chemical agents on rice husk activated carbon has created new spectra assumed to have new surface functional groups. A wide transmittance band at maximum at 3427.58 cm-1 is assigned as O-H stretching vibration of hydroxyl group as a result of modification phosphoric acid. A band at 1633.04 cm-1 is generally attributed to C=O stretching vibration in carboxyl groups ketones, aldehydes or lactones and it is also associated to C=C stretching vibration in aromatic rings. A weak band at 1382.32 cm-1 is attributed to C-H asymmetries bending. A medium band 1097 and 1068.02 cm-1 are ascribed to C-O stretching vibration in ester and ionized linkage P-O in acid phosphorous ester. These bands are also due to the Si-O-Si asymmetric stretching vibration [18]. The presence of band at 796.05 cm-1 and 793.17 are ascribed to C-H in out-of-plane bending in the edges of aromatic rings and Si-OH stretching vibration. The band at 464.57 is assigned to Si-O group. This functional group indicates that rice husk activated carbon contain silicate elements [19].
 |
Figure 3: ( A) FTIR spectra of rice husk activated carbon without chemical agent (B) FTIR spectra of rice huisk activated carbon with ZnCl2/H3PO4. Click here to View figure |
Surface Morphology of rice Husk Activated carbon
Fig. 4 shows the morphology structure and elemental analysis of rice husk activated carbon. The image shows irregular external surface with cracks and crevices into various size of pores. It seems that the forming of cavities of rice husk activated carbon was resulted from the initiation and evaporation process of chemical agent during the pyrolysis process. However, the silicate and zinc are not easily removed in this process which was assumed due to the low temperature. The surface of particles is most likely covered with silicate which is supported with the highest silicate content. The elemental analysis of rice husk activated carbon was determined by EDX measurement. The relative composition is 22.2 % of silicon, 58.1 % of oxygen, 5.5 % of chlorine, 9.2% of carbon and 5.2% of zinc. This result is relevance with the FTIR data consisting of the Si-O functional group.
 |
Figure 4: Surface morphology and elemental analysis of rice husk activated carbon. Click here to View figure |
Application of rice husk activated carbon on the Adsorption process
Effect of adsorbent dosage
Fig. 5 shows the effect of adsorbent dosage on percentage of 2-cholorophenol removal at pH 7 and room temperature. The shape of adsorption capacity increases with increasing the adsorbent dosage. This phenomenon is due to the fact that as the adsorbent dosage increases, the number of sorption sites of adsorbent such as pore volume and surface area are available for 2-chlorophenol adsorption.
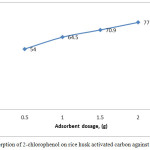 |
Figure 5: Adsorption of 2-chlorophenol on rice husk activated carbon against adsorbent dosage. Click here to View figure |
Effect of pH
Fig. 6 shows that the effect of pH can influence the adsorption capacity of 2-chlorophenol. It was observed that the percentage of removal of 2-chlorphenol increased as pH solution increased. The 2-chlorophenol uptake sharply increases at pH 4 to 5, remains constant from 5 to 7 and decreases at pH 8. The maximum sorption was obtained at pH 7. The adsorption process of 2-chlorophenol could be explained by two methods: chemisorption and physisorption. At lower pH, 2-chlorophenol solution had acid condition with the excess positive charges, H+ ions on the surface of activated carbon while the rice husk activated carbon have active site with negative charges. Interaction and repulsion between positive and negative charges caused a neutralization ions and result the increasing of the removal of 2-cholorophenol. At higher pH 8, surface of adsorbent has high the negative charges. The excess negative charges in the solution resulted repulsion between 2-chlorophenol and adsorbent which caused reducing of 2-chlorophenol removal
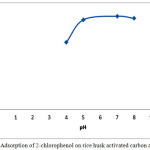 |
Figure 6: Adsorption of 2-chlorophenol on rice husk activated carbon against pH. Click here to View figure |
Effect of Contact Times
The effect of contact times is one of the important roles to determine the adsorption capacity. The possible reasons are its effect on the chemical reaction that occurs between adsorbate and surface area of adsorbent. Fig. 7 shows the relationship between contact time and the percentage adsorption from solution with rice husk activated carbon. The removal of 2-chlorophenol have trend to increase as contact time increase reaching to a maximum value at 45 minutes and decrease after 45 minutes. The increasing adsorption capacity was due to the fact that increasing contact time enhanced the diffusion or transportation adsorbate to the pores starting from external to the internal site of the adsorbent. The increasing contact time to 60 min show the decreasing of the adsorption of 2-chlorophenol. It is assumed that the reduction of adsorption capacity was caused by the fulfill of adsorbate into pores during adsorption process.
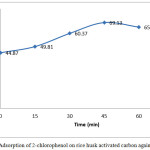 |
Figure 7: Adsorption of 2-chlorophenol on rice husk activated carbon against contact time. Click here to View figure |
Adsorption Isotherms
The equilibrium adsorption is usually described by the amount of adsorbate on the adsorbent using models of the Langmuir and Freundlich isotherms. Once the surface site is filled, no further sorption can take place at that site. This phenomenon indicates that the surface chemistry of the adsorbent reaches a saturation point where the maximum adsorption of the surface was achieved.
The Langmuir isotherm model was carried out using the following equation:
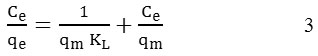
Where Ce is the concentration of adsorbate at equilibrium in solute (mg/L), qe is the adsorption capacity at equilibrium solute concentration (mg/g), qm is the maximum adsorption capacity relating to monolayer coverage (mg/g), KL is the Langmuir constant related to affinity of point of (L/mg).
Fig. 8 shows the linear plot (R2 0.9784) of specific adsorption (Ce/qe) versus equilibrium concentration (Ce) indicated the results the Langmuir model.
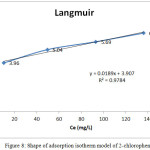 |
Figure 8: Shape of adsorption isotherm model of 2-chlorophenol. Click here to View figure |
The Freundlich isotherm model follows the equation based on the multilayer of adsorbent. The Freundlich equation is expressed as following equation:
![]()
The linear plot of the Freundlich isotherm model is given as the following equaltion.
![]()
Where KF is the Freundlich constant related to several environmental factors , n is the parameter presenting the energy heterogeneity of the adsorption sites. Fig. 9 shows a plot linier of log qe versus log Ce shows the the Feundlich isotherm for adsorption. The values of KF and n are obtained from both intercept and slope, respectively. The parameter n is usually follow the value range at 1<n<10 relating to the favorable adsorption process. The Langmuir and Freudlich isotherms were plotted in Table 2.
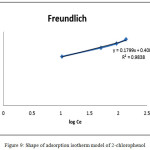 |
Figure 9: Shape of adsorption isotherm model of 2-chlorophenol. Click here to View figure |
Table 2. Langmuir and Freundlich isotherm for 2-chlorophenol adsorbed.
| Adsorbent |
Langmuir isotherm model |
Freundlich isotherm model |
||||
|
qm (mg/g) |
KL (L/mg) |
R2 |
KF |
n |
R2 |
|
| ZnCl2/H3PO4 |
67,567 |
0,0818 |
0.9784 |
2,5586 |
5,5866 |
0.9838 |
Table 2 illustrates the Langmuir and Freundlich isotherm models for the adsorption of 2-chlorophenol. Based on the experimental data, both Langmuir and Freundlich isotherm model show best fitting line with R2 values 0.9784 and 0.9838, respectively. These phenomena indicate that the rice husk activated carbon possess a well adsorption behavior of 2-chlorophenol.
Adsorption Kinetic Modeling
The adsorption kinetics is important because the kinetics can describe an uptake rate of adsorbate relating to the adsorption mechanism. In this study, the effect of contact time was applied in order to describe the uptake rate of 2-chlorophenol at different times. To investigate the adsorption kinetics were applied to the model of the pseudo-first-order and pseudo-second-order models. The linear plots of these equations can predict whether the model suitably as an adsorption process or not. The expression for pseudo-first-order equation model is shown in following equation (4):
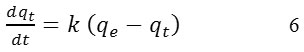
where qe and qt are the sorption capacities at equilibrium at time t, respectively (mg/g), k is the pseudo-first-order sorption rate constant ( L/min). after integration, from t = 0 to t = t and qt = 0 to qt = qt the equation becomes:
![]()
The adsorption kinetics of pseudo-second-order models is based on the sorption capacity of the adsorbate. The rate equation is expressed as:
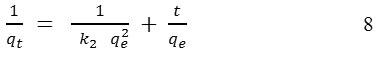
The adsorption capacity shows as qe and qt (mg/g) are at equilibrium and at time t, respectively, k2 is the rate constant of the pseudo-second-order .
The experimental data for a plot of log (qe-qt) against time for first order kinetics have poor fitting line with relative low of R2 value. The obtained yield shows that the first order kinetics is not appropriate for describing the adsorption kinetics process of 2-chlorophenol by the rice husk activated carbon. The pseudo-second-order rate equation was determined from the slope and intercept of the plot of t/qt versus time (t) which is shown in Fig. 10. In this study, a linear plot found in the experiment data show a best fitting line with high correlation coefficient R2 = 0.9976. It can be noted that the pseudo-second-order model described satisfactorily the adsorption of 2-chlorophenol by the rice husk activated carbon. However, from the calculation for pseudo-first-order equation, the best fitting line with correlation coefficient R2 = 0.0449 indicates that the kinetics of adsorption 2-chlorophenol was not satisfied to be described.
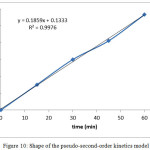 |
Figure 10: Shape of the pseudo-second-order kinetics model. Click here to View figure |
Conclusions
The preparation of activated carbon from waste of rice husk was successfully investigated. The nitrogen adsorption and desorption isotherm shows Type IV isotherms with predominantly mesoporous structure with multilayer adsorption. BET and Langmuir surface areas were 144.6 m2g-1 and 212.5 m2g-1, respectively. The adsorption capacity of 2-chlorophenol was influenced by different parameters. The equilibrium adsorption isotherm can be described by both the Langmuir and the Freundlich isotherms model with best fitting line. The values of R2 shows an excellent fitting line for the pseudo-second–order with correlation coefficient R2 = 0.9976 indicating the satisfactory pseudo-second-order model. Finally, rice husk activated carbon was effectively used as adsorbent for removal of 2-chlorophenol.
Acknowledgements
The research was been funded by DPPM and Chemistry Department, Mathematic and Natural Sciences Faculty, Islamic University of Indonesia (UII), Indonesia.
References
- Yakout, S.M., Sharaf El-Deen, G, Arabian Journal of Chemistry, 2016. 9: . 155-162.
CrossRef - Haydar, S., Ferro-Garcıa, M.A., Rivera-Utrilla, J., Joly, J.P., Carbon, 2003. 41: . 387-395.
CrossRef - Jinping, Z., Wencai, R., Hui-Ming, C, Journal of Materials Chemistry, 2012. 22: 20197–20202.
CrossRef - Wang, J.P., ; Feng, H. M; Yu, H. Q, . J. Hazard. Mater., 2007. 144:. 200-207.
CrossRef - Allaboun, H., Abu Al-Rub, F.A, Material, 2016. 9(4): 251.
CrossRef - Igbinosa, E.O., Odjadjare, E. E., Chigor, V. N., Igbinosa, I. H., Emoghene, A. O., Ekhaise, F. O., Igiehon, N. O., Idemudia, O. G., Hindawi Publishing Corporation The ScientificWorld Journal, 2013. 2013.
- Radhika, M., Palanivelu, K., J. Hazard. Mater., 2006. 138.,116-124.,.
CrossRef - Liu, Q.S., Zheng, T., Wang, P., Jiang, J.P., Li, N., Chemical Engineering Journal Hazardous Material, 2010. 157(2-3): 348-356. Mohan, D., Singh, K.P., Singh, V.K, Carbon, 2008. 152: 1046-1053.
- Kumar, S.S., P.; Dhankhar, R. M. V.; Bidra, S., Research Journal of Chemical and Environmental Sciences, 2013. 1(5): 126-129.
- Montes-Morán, M.A., Suárez, D., Menéndez, J. A., Fuente, E Carbon, 2004. 42: 1219-1225.
CrossRef - Allwar, IOSR Journal of Applied Chemistry 2012. 2: . 9-15.
- Kennedy LJ, V.J., Kayalvizhi K, Sekaran G., Chem. Eng. J, 2007. 132: . 279-287.
CrossRef - Kilic M., A.-V.E., Putin A. E., Journal of Hazardous Materials, 2011. 189: 397- 403.
CrossRef - Mouhamed, E.K.S., Ramzi, K., Elimame, E., Younes, M., Arabian Journal of Chemistry, 2014. 7: 109-113.
CrossRef - Sing, K.S.W., Everett, D.H., Haul, R.A.W., Moscow, L., Pinerotti, R.A., Rouquerol, J., Siemieniewska, T., Pure Appl. Chem, 1985. 57: 603.
CrossRef - Dougall, G.J.M., J.S.Afr.Inst.Min.Metall., 1991. 91(4): 109-120.
- Ghorbani, F., Sanati, A. M., Maleki, M, Environmental Studies of Persian Gulf 2015. 2(1): p. 56-65.
- Abdel Rahim, M.M., Ismail, M. M., Abdel Mageed, A. M. , International Journal of Advanced Research, 2015. 3(2): p. 491-498.

This work is licensed under a Creative Commons Attribution 4.0 International License.









