Removal Bismarck Brown G dye from Aqueous Solution over a Composite of Triazole-Polyvinyl Chloride Polymer and Zinc Oxide
Nour Abd Alrazzak1,Saadon Abdulla Aowda2 and Abbas Jassim Atiyah2
1University of Babylon, College of Science of Women's, Department of Chemistry, Hilla, Post Box 4.
2University of Babylon, College of Science, Department of Chemistry, Hilla, Post Box 4.
Corresponding Author E-mail: abbaslafta2009@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330541
The present study involves synthesis a composite of triazole-poly vinyl chloride (PVC) polymer with nano- crystalline zinc oxide triazole-PVC/ZnO. The produced composite was characterized using fouier infrared spectroscopy(FTIR), X-Rays diffraction(XRD) and micro- elemental analysis (CHN). The activity of the produced composite with respect to neat zinc oxide was investigated by following both of adsorption and photocatalytic removal of Bismarck Brown G (BBG)dye from their aqueous. The obtained results in this study showed that triazole-PVC/ZnO was more efficient than neat ZnO on dye removal for both of adsorption and photocatalytic reaction.
KEYWORDS:Zinc oxide; Composite materials; Thiazine polymer; Adsorption processes; Dye removal from aqueous solutions
Download this article as:| Copy the following to cite this article: Alrazzak N. A, Aowda S. A, Atiyah A. J. Removal Bismarck Brown G dye from Aqueous Solution over a Composite of Triazole-Polyvinyl Chloride Polymer and Zinc Oxide. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Alrazzak N. A, Aowda S. A, Atiyah A. J. Removal Bismarck Brown G dye from Aqueous Solution over a Composite of Triazole-Polyvinyl Chloride Polymer and Zinc Oxide. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=38705 |
Introduction
In last few years nano materials have much attention and are become a topic research over our world due to their massive importance in our modern life. Among wide variety of these Nano scale materials1-3, Nano crystalline zinc oxide is very important photocatalytic material that is used currently in wide range of applications in different fields4. These involve gas sensors, solar cells, semiconducting photocatalyst, industrial catalyst, and electrical and optical devices. Beside these applications , zinc oxide was more attractive photocatalytic material due to its excellent physical and chemical properties, such as thermal and chemical stability, no toxicity, relatively low cost as well as it is capable of recovery with high efficiency to be recycled after each use after very simple reactivation processes5,7. Zinc oxide can be excited thermally and/or photoexcited via absorption of light with energy that is equal to or greater its bandgap( hν≥Eg) which is equal to 2.31 eV8-10. Upon excitation, its produced (h+ , e-) pairs on both valence band and conduction band respectively. These species then are migrated to the surface to participate on redox reactions with pre- adsorbed species on the surface. The main drawback in this point is the recombination reaction that occurs normally between conduction band electrons and valence band holes11-13. This process would affect negatively on the activity of zinc oxide as a photocatalyst. So that the goal point here is how to prevent or at least reduce the rate of recombination reaction. Different methods can be applied to perform the photocatlytic activity of zinc oxide and increase the time of charge separation of (h+ , e-) pairs14,15. These involves surface modification by coupling with another photocatalyst with smaller bandgap, doping of some reactive metal with ZnO, surface sensitization, doping with some of non-metals16. In addition to these method, zinc oxide surface can be modified by combination with polymeric materials by absorption or reaction with dispersed polymer on its surface17.
The present study describe modification of the activity of zinc oxide by combination with polytriazole to yield triazole-PVC/ZnO and the activity of the formed composite would be investigated by following removal of BBG dye over surface of neat zinc oxide and triazole-PVC/ ZnO.
Experimental
Chemicals
Poly vinyl chloride 99.9% Sigma Aldrich Company, Pyridine 99.8% sigma Aldrich company, tetra hydro furan 99.5% sigma Aldrich company.
Using Zinc Oxide
Using zinc oxide was ZnO nanoparticle (ZnO, Fluka company, 99.5%) with average nanoparticle of 25-30 nm. The dye used was Bismarck brown G (BBG- Sigma Aldrich). It has a molecular formula (C21H24N8.2HCl). This dye has a maximum absorption at a wavelength of 468 nm and it was obtained from Al-Hilla Textile Factory.
Synthesis of Triazole-PVC Polymer
4- Hydrazino-5- methoxy cinnolin (0.0304 gm, 0.0016 mole) was added to (0.125g) poly (vinyl chloride) and few drops of pyridine in (50mL) tetrahydrofuran (THF) . A brown prcipitate was formed after refluxing the mixture for five hours .The modified polymer (P1) was filtered,washed with redistilled water,methanol,ether and dried under vacuum.(0.00057 mole, 0.2g) of 1-((4-(5-mercapto-4H-1,2,4-triazol-3-yl)phenyl)diazenyl)naphthalen-2-ol [N1] was added to (0.1g) of poly (vinyl chloride) and (3) drops of pyridine in (15)ml THF, which reflux for (5) hours, the solvent was evaporate and the orange solid product was obtain18. Schematic description for polymer synthesis is shown in Fig. 1.
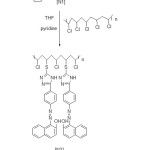 |
Figure 1: Schematic description for the route for synthesis of modified triazole –PVC polymer Click here to View figure |
Synthesis of a Composite of Modified Triazole- PVC/ZnO
A composite of P/ZnO was synthesized as follows: 1 gm. of zinc oxide nanoparticles was suspended in 100 ml of distilled water with continuous stirring at 30 ºC for 60 minute. In another flask 0.02 gm of triazole polymer was suspended in 100 ml of distilled water with continuous stirring at 50 ºC for two hours. Then these two suspended solutions were mixed together in one flask with continuous stirring for four hours at 35 ºC under normal atmospheric conditions. Then the obtained mixture was left aside for two hours to cool to room temperature and the it filtered out. The precipitate washed with water for several times and the obtained solid was dried in oven at 110 ºC for overnight. The obtained composite was investigated using FTIR spectroscopy, XRD patterns, and CHN microelemental analysis.
Adsorption and Photocatalytic Activity of Triazole-PVC/ZnO
The activity of both neat zinc oxide and the composite P/ZnO was investigated via removal of BBG dye from aqueous solution by adsorption and phtocatalytic reaction. To perform this part a series of experiment were carried out using solution of dye 50 ppm . This was suspended in a reaction cell in 30 ml in a homemade phtoreactor as shown in Fig. 2. Adsorption processes were carried out in 30 mL conical flask using a thermal controlled shaker water bath under air conditions. For each experiment, a sample containing a dye concentration of 50 ppm in 30 mL was suspended with a desired mass of a used catalyst (neat ZnO and triazole-PVC/ZnO) . All adsorption processes were conducted for one hour for each run, periodically 1 mL of the reaction mixture was withdrawn for each 15 minutes for each experiment19. Samples then were centrifuged for several times and the absorbance of the supernatant obtained liquid was recorded at a wavelength of 468 nm using UV-Visible Spectrophotometer Shimadzu 1650 PC-UV-visible. The efficiency for dye removal from the simulated industrial wastewaters (R%) was calculated using the following relationship19 :
Removal%= Ci– Ct/Ci x100
Where, Ci is the initial dye concentration and Ct is the dye concentration that is remaining at interval time of adsorption process.
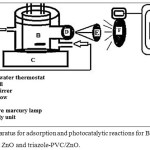 |
Figure 2: Apparatus for adsorption and photocatalytic reactions for BBG dye removal over both neat ZnO and triazole-PVC/ZnO. Click here to View figure |
Photocatalytic activity of ZnO and triazole-PVC/ZnO was investigated by following phtocatlytic removal of BBG dye over theses catalysts. To perform that, a series of experiments were carried out under different reaction conditions . These experiments were performed using a solution of BBG dye with a suspension of ZnO and triazole-PVC/ZnO. All reactions were carried out in the homemade photoreactor that is shown in Fig. 2 above. For each experiment, same amount of dye was used (30 mL, 50 ppm) over a suspension of the used ZnO or triazole-PVC/ZnO) catalysts (0.10 gram). Reaction mixture was mixed using magnetic stirrer for 20 minute in the dark for each run in order to approach adsorption equilibrium. Then irradiation was flushed with UV radiation using candles (Philips) 70 watt UV. Radiation. Then periodically, at each 15 minute by withdrawing of 1 mL of the reaction mixture. This withdrawal sample was centrifuged carefully at 5000 rpm. The photocatalytic BBG dye removal over each of ZnO and triazole-PVC/ ZnO was calculated by recording the absorbance of the supernatant liquid at a wavelength of 468 nm with Shimadzu 1180 UV-Vis Spectrophotometer.
Fourier Transform Infrared Spectroscopy (FTIR)
Surface functional groups of both of ZnO and triazole-PVC/ZnO were conducted using FTIR spectroscopy. In all runs, FTIR spectra were investigated using FTIR Perkin Elminer Spectrophotometer. All spectra were recorded from 400 to 4000 cm-1. Samples were dried carefully and then mixed with KBr powder to make them as pellets prior to perform run.
X-rays Diffraction (XRD )
Crystal structure of both ZnO and triazole-PVC/ZnO was investigated with XRD, Simadzu-6000 X-rays diffractmeter . The used XRD diffractmeter was provided with a nickel filter, and the radiation source was CuKα radiation at 40 kV and 30 mA. All XRD patterns were recorded in the range of 2Theta= 20- 80 deg.
Microelemental Analysis (CHN)
Microelemental analysis for each of ZnO and triazole-PVC/ZnO samples was investigated to estimate the content of C,N and H content for these materials using EuroEA3000Elemental Analyzer, Italy.
Results and Discussion
Polymer and Catalyst Characterization
Fourier Transform Infrared Spectroscopy for Triazole-Pvc and the Composite
A modified PVC/triazole polymer was synthesized as described above. The formed polymer was investigated using FTIR specrum as shown in Fig. 3. Weak band around 635 cm-1 can be assigned to (C-Cl) vibration modes. The band around 1554 cm-1 indicated that the end groups of the Polymer is C=C. The band around 2920 cm-1 can be assigned to aliphatic (C-H).The band around 3070 cm-1 is related to C-H aromatic and that around 2936 cm-1 C-H aliphatic20. The band around 1569 cm-1is assigned to C=C, and that around 1648 cm-1 is related to C=N. The band around 3379 cm -1 is assigned to N-H Stretching mode, and that around 1457 is related to C-H bending of –CH2. The band around 737 C-H bending of ortho substituted benzene ring. The band around 3448 cm -1 is assigned to OH stretching modes20. The band around 3321 cm -1 is attributed to NH stretching modes. The band around 3078 cm -1 can be assigned to the aromatic CH stretching modes. The band around 1554 cm -1 is assigned to C=N stretching modes. The band around 1485 cm -1 is attributed to C=C aromatic stretching modes. The band around 1342 cm -1 is related to C-N stretching modes21.
FTIR spectra for triazole-PVC/ZnO composites are shown in Fig. 4. From these spectra it can be seen that, signals of triazole-PVC are almost appeared in FTIR spectra of the composites. From these spectra, the bands that appear 500-650 cm –1 are assigned to metal oxide (Zn-O) bonds22,23. The bands at the range of 950-1480 cm-1 are attributed to the presence of stretching and bending of oxygen at the surface triazole-PVC/ZnO composite. bands around 1640 cm-1 and that around 3445 cm-1 are assigned to the stretching modes of OH groups on the surface of triazole-PVC/ZnO composite. The band around 3400 cm-1 is assigned to vibration mode of O-H group the composite surface24,25.
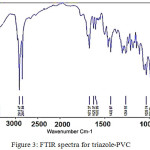 |
Figure 3: FTIR spectra for triazole-PVC Click here to View figure |
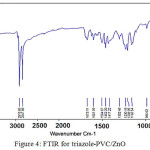 |
Figure 4: FTIR for triazole-PVC/ZnO Click here to View figure |
X-Rays Diffraction(XRD)
XRD patterns for both ZnO and triazole-PVC/composites are shown in Figs. 5 and 6. From these XRD patterns for neat and modified ZnO with triazole –PVC, it can be seen that, the main peaks for ZnO are appeared in two cases and it is showed wurtzite type hexagonal structure; ( Joint card of powder diffraction standards, JCPDS 36−1451 card) for ZnO samples26,27. The peaks at 2θ of 31.7°, 34.5°, 36.2°, 47.5°, 56.5°, 67.94, 69.08, 72.5 and 76.95 are corresponded to the crystal planes (100), (002), (101), (102), (110), (112), (201), (004) and ( 202) of crystalline ZnO. This means that doping zinc oxide with triazole-PVC in this ratio doesn’t affect its crystallite structure. For this reason XRD patterns of triazole-PVC/ZnO almost showed same patterns for neat zinc oxide26,27.
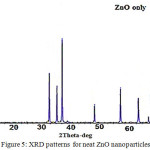 |
Figure 5: XRD patterns for neat ZnO nanoparticles Click here to View figure |
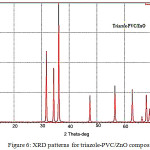 |
Figure 6: XRD patterns for triazole-PVC/ZnO composites Click here to View figure |
Microelemental Analysis (CHN)
Micoelemental content of neat ZnO and triazole-PVC/ZnO samples was investigated using CHN microelemental analysis and the obtained results are shown in Table 1. From these results it can be seen that there is a clear evidence for the formation of the proposed composite by presence these elements that composed the polymer on the matrix of zinc oxide.
Table 1: Microelemental results for both neat ZnO and triazole-PVC/ZnO
| Sample | C% | H% | N% |
| ZnO neat | nil | 0.01 | nil |
| Triazole-PVC/ZnO | 0.18 | 0.02 | 0.06 |
Adsorption Ability of BBG Dye over ZnO and Triazole-PVC/ZnO
Adsorption ability of both neat zinc oxide and the prepared composite was investigated by investigating removal of BBG dye over ZnO and triazole-PVC/ZnO. The obtained results are presented in Figs. 7 and 8. From these results it can be seen that, the activity of triazole-PVC/ZnO was more efficient than neat ZnO in BBG dye removal from aqueous solution by adsorption process. This result can be attributed to enhancement surface properties of ZnO by doping with triazole-PVC. This can lead to increase porosity of the surface and this leads to increase its adsorption capacity for the composite in comparison with neat ZnO. The effect of temperature on adsorption efficiency of BBG dye over both neat ZnO and the composite, a series of experiments were carried out using same reaction conditions at a range of temperatures from 288 to 303 K. The obtained results are shown in Figs. 7 and 8 28. From these results it can be seen that the efficiency of dye removal was increased with increase in the temperature of reaction mixture for using both of neat ZnO and the composite and the maximum removal efficiency was obtained at 303 K. These results can be attributed to the effect of temperature on the diffusion of BBG dye molecules from bulk solution into the adsorption sites on the surface of both ZnO and the composite 21. Elevation in temperature of reaction mixture can lead to increase the rate of diffusion of dye molecules and this leads to increase the efficiency of dye removal upon increasing adsorption temperature in this range29,30.
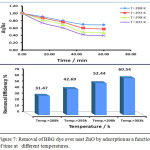 |
Figure 7: Removal of BBG dye over neat ZnO by adsorption as a function of time at different temperatures. Click here to View figure |
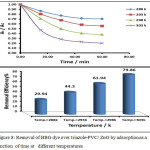 |
Figure 8: Removal of BBG dye over triazole-PVC/ ZnO by adsorption as a function of time at different temperatures Click here to View figure |
Photocatalytic Removal of BBG Dye over ZnO and Triazole-PVC/ZnO
The photocatalytic activity of neat ZnO and triazole-PVC/ZnO was investigated by following removal of BBG dye over a suspension of these materials. To perform that, a series of experiments were carried out and for each experiment 0.05 g. of the used materials were suspended in 30 mL of 50 ppm of BBG dye with continuous stirring under normal air conditions at range of temperatures from 288 to 303 with constant interval increment of 5 degree for each successive runs. Before starting light reaction, dark reaction was undertaken by stirring reaction mixture for around 15 minute in dark to reach adsorption equilibrium. After that, photocatalytic reaction was initiated by irradiation with UV light from low pressure mercury lamp. The reaction temperature was controlled at a desired value using a thermostatic circulation water jacket. The obtained results of photocatalytic removal of BBG dye over a neat ZnO are shown in Fig. 9 and that for triazole-PVC/ZnO are shown in Fig. 10 respectively.
From these results, it can be seen that using triazole-PVC/ZnO as a catalyst for dye removal was more efficient in comparison with use neat ZnO under applying the same reaction conditions. Better removal efficiency of BBG dye after one hour over composite was around 82% while it was around 6% over neat ZnO31,32. This significant enhancement in dye removal over composite can be attributed to the synergistic effect of triazole-PVC and ZnO. . Besides that, presence of triazole-PVC particles in this composite can reduce recombination reaction between conduction band electrons ( e–CB) and valence band holes (h+VB) by increasing separation between these species33. For neat ZnO, recombination reaction occurs with high rate which leads to reduce the efficiency of the photocatalytic process. For composite, these species can migrate to the surface and then participate in redox reactions with the pre-adsorbed species on the surface of the coposite34. Also from the obtained results for both of neat ZnO and the composite it can be seen that the efficiency of BBG dye removal was increased with increase of temperature. It is well known that phtocatalytic reactions are not too sensitive towards elevation in reaction temperature and it depend mainly on electron transfer from valence band into the conduction band through structure of particles of the catalyst. It is believed that enhancement in the efficiency of dye removal with increasing of reaction temperature is probably attributed to the effect of temperature on the diffusion of dye molecules from bulk solution to the surface and its effect on adsorption-desorption processes for reacting species35,36.
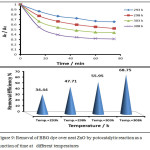 |
Figure 9: Removal of BBG dye over neat ZnO by potocatalytic reaction as a function of time at different temperatures Click here to View figure |
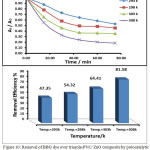 |
Figure 10: Removal of BBG dye over triazole-PVC/ ZnO composite by potocatalytic reaction as a function of time at different temperatures Click here to View figure |
Conclusions
From the obtained results in this study it was found that doping zinc oxide with triazole-PVC improved its surface properties. The composite triazole-PVC/ZnO showed higher adsorption ability and photocatalytic activity in BBG dye removal from aqueous solution in comparison with using neat zinc oxide.
References
- Wang Z, Huang C, Huang Y, Hou Y, Xie P, Zhang B, Cheng H, Chem. Mater.,2001, 13, 678-682.
CrossRef - Xu J, Pan Q, Shun Y, Tian Z, Sens. Actuators, B. Chem., 2000, 66, 277-279.
CrossRef - Wu J, Xie C, Bai Z, Zhu B, Huang K , Wu R, Mater. Sci. Eng. B, Solid- State Mater. Adv. Technol., 2002, 95 ,157-161.
- Curridal M, Comparelli R, Cozzli P, Mascolo D, Mater. Sci. Eng., C, Biomin. Mater., Sens. Sys., 2003,23 ,285-289.
- Shanthi M, Kuzhalosai V, India Journal of Chemistry, 2012, 51A ,428-434.
- Garg K, Amita M, Kumar R, Gupta R, Dyes and Pigments, 2004 ,63 (3), 243-250.
CrossRef - Amjed M O, Ahmed S F, Abbas J L, International Journal of Science and Research, 2014, 3(11), 2133-2138.
- Dezhongyu J, Ruzuicai M, Zhihongliu R, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2004, 60(7), 1617-1624
CrossRef - Attia A , Kadhim S , Hussein F , E- Journal of Chemistry, 2008, 5(2), 219-223.
CrossRef - Vinu R, Mudras G, Journal of the Indian Institute of Science, 2010,90(2), 189-230.
- Katarzyna S, Filip C, Magdalena N, Teofil J, Journal of Nanomaterial, 2012, 2012,1-19.
- Huang M, Mao S, Feick H, Science, 2001,292, 1897-1909.
CrossRef - Vayssieres L, Advanced Materials, 2003, 15,464-466.
CrossRef - Nitish R, Kam T, Debabrata P, The Journal of Physical Chemistry C, 2015 , 119 (33),19117–19125.
CrossRef
- Nitish R, Yohan P, Youngku S, Kam T, Debabrata P, Applied Materials Interfaces, 2014,6 (19), 16498–16507
CrossRef - Nakajima N, Kato H, Okazaki T, Sakisaka Y, Surface Science, 2004 ,561(1), 93-100.
CrossRef - Hong R, Qian J, Cao J, Powder Technology, 2006 ,163, 160-168.
CrossRef - Ulbrich K, Ilavský M, Dušek K , Kopeček J, European Polymer Journal, 1977,13(7), 579-585.
CrossRef - Ameri A, Esrafily A, Iranian Journal of Environmental Health Sciences & Engineering, 2013 , 10(19), 1-9.
- Murti Y, Agnihotri R, Pathak D, American Journal of Chemistry, 2011,1(2), 42-46.
CrossRef - Olfa A, Radha B, National Journal of Chemistry, 2010, 3, 74- 85.
- Jun S, Kim S, Han J, Journal Korean Ceramic Society,1998, 35(3), 209-215.
- Taps A, Majewaski P, Aldinger F, Journal of the American Ceramic Society, 2000 ,83 (12), 2954-2960-
CrossRef - Gouvea C, Wypych K, Moraes S, Duran N, Nagata N , Zamora P, Chemosphere, 2000, 40, 440-443.
- Bourikas K, Stylidi M, Dimitris K , Verykios X, Langmuir, 2005, 21(12), 9222–9230.
CrossRef - Ibhadon O, Fitzpatrick P, Catalysts, 2013, 3 ,189-218.
CrossRef - Friedmann D, Mendive C, Bahnemann D, Applied Catalysis B: Environmental, 2010, 99, 398–406.
CrossRef - Ahmed H, Abbas L, Zahra A, Falah H, Asian Journal of Chemistry; 2014,26, 167-172.
CrossRef - Matos J, Laine J, Herrmann M, Uzcategui D, Brito L, Applied Catalysis B: Environmental, 2007 ,70, 461-469.
CrossRef - Ashri W, Daud W, Houshamnd A, Journal of Natural Gas Chemistry, 2010,19, 267– 279.
CrossRef - Rusheng Y, Rogngbo G, Jingtang Z, Scripta Materialia, 2005,62 ,1329-1334.
- Ahmed K, Falah H, Ahmed H, Bahnemann D, International Journal of Photoenergy, 2014, 2014, 2-9.
- Sobana N; Swaminathan M, Solar Energy Materials and Solar Cells, 2007, 91, 727-734.
CrossRef - Gokona N; Hasegawa N, Kaneko H, Aoki H, Tamaura Y, Kitamuara M, Solar Energy Materials and Solar Cells, 2003, 80 ,335-341.
CrossRef - Suna H, Yong K, Suna X, Donga Y, Materials Chemistry and Physics, 2009,115(1), 303-308.
CrossRef - Movahedi M, Mahjoub R, Darzi J, Journal of the Iranian Chemical Society, 2009,6(3), 570-577.

This work is licensed under a Creative Commons Attribution 4.0 International License.









