Phytochemical and in Vitro Antioxidant Activity of Various Bark Extracts of Syzygium Cumini (L).
Venkateshan Narayanan1, Rajapandi Raju1, Vuppalapati Lavakumar2, Amutha Iswarya Devi Jeyaraman1 and Arunpandiyan Jeyakumar1
1Department of Pharmaceutical Chemistry, Arulmigu Kalasalingam College of Pharmacy, Krishnankoil - 626126. Tamilnadu.
2Department of Pharmaceutics, Arulmigu Kalasalingam College of Pharmacy, Krishnankoil - 626126. Tamilnadu.
Corresponding Author E-mail: akcpprl@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330551
Syzygium cumini (L) (S .cumini) commonly known as jamun belongs to the Myrtaceae family. The aim of the present study includes phytochemical investigation and in vitro anti-oxidant capacity of various crude extracts from the bark of syzygium cumini (L) by various anti-oxidant assays namely DPPH (2, 2-diphenyl-1-picrylhydrazyl), Nitric Oxide (NO) and Hydrogen peroxide (H2O2). Chloroform (CHCl3), Ethyl acetate (EA) and Methanolic (MeOH) extracts of S. cumini gave positive results for steroids, alkaloids, tannins and flavonoids. The scavenging ability of chloroform, ethyl acetate and methanolic extracts along with standard (Ascorbic acid) were evaluated between the range of 20µg/ml to 300µg/ml using DPPH anti-oxidant assay and the IC50values were found to 41µg/mL, 57µg/mL, 53µg/mL and 6.1µg/mL respectively. To prove further its anti-oxidant activity, they were evaluated using NO and H2O2 antioxidant assays.
KEYWORDS:Anti-Oxidant Activity; DPPH; Phytochemical Screening and Syzygium Cumini (L)
Download this article as:| Copy the following to cite this article: Narayanan V, Raju R, Lavakumar V, Jeyaraman A. I. D, Jeyakumar A. Phytochemical and in Vitro Antioxidant Activity of Various Bark Extracts of Syzygium Cumini (L). Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Narayanan V, Raju R, Lavakumar V, Jeyaraman A. I. D, Jeyakumar A. Phytochemical and in Vitro Antioxidant Activity of Various Bark Extracts of Syzygium Cumini (L). Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=37380 |
Introduction
In the modern work culture, there are numerous reasons that lead to oxidative stress in body which is challenging to cope with and leads to many disorders like early aging and age related neuro degenerative disease. Imbalance between oxidants and antioxidants causes oxidative stress. Free radical is defined as any atoms (e.g. oxygen, nitrogen) which have at least one unpaired electron in the outermost shell, and is accomplished of independent subsistence. Oxygen is the most significant element for life which is the major resource of free radicals. Oxygen is used by cells for generating energy, which leads to free radical generation in the end of ATP (adenosine triphosphate) production by the mitochondria. The free radicals play a twin role, both as toxic and beneficial compounds. Formation of free radicals at a lower or moderate levels, contribute to good cellular responses and immune functions in human health and development. Free radicals occur not only in normal cellular process but also generated due to certain external factors like chemicals (polycyclic aromatic hydrocarbon, cadmium, lead, etc.), radiation, smoking and high fat diet.A balance between formations of free radicals and its detoxification is essential for normal cellular function. When this balance gets disturbed due to any reason, it leads to cellular damage because of excess of free radical generated. The presence of excessive free radical is termed as oxidative stress. The free radicals can cause genetic instability by reacting with DNA which results in cancer1, mutation, circulatory disturbance and early aging2-5. This makes researchers a way to work on this field and come out with entities which have good antioxidant activity and can be used to relieve the oxidative stress and help in maintaining good human health.Synthetic antioxidants have been reported to exhibit higher toxicity to humans, which makes it a need to look for natural source (herbs) for a good antioxidant candidate. Recent research works has confirmed this, as some of the medicinal plantsare having good therapeutic anti-oxidant activity. Among them number of naturally occurring antioxidants; ascorbic acid, carotenoids and phenolic compounds are successful in scavenging reactive oxygen species (ROS) by inhibiting lipid peroxidation.
The S. cumini (L.) Skeels (Syns. Syzygium jambolana DC, Eugenia cumini Druce, Eugenia jambolana Lam.), commonly known as Jamun, belongs to the family Myrtaceae or Myrtle6. The other common names are Indian blackberry, Java plum, Jambu, black plum and Jambuletc. The jamunfruit as well as different parts of the plants have high medicinal values, possess varied uses to mankind.The various extracts of different parts of S. cumini possess a range of pharmacological properties such as antibacterial7-9, antimicrobial10, antifungal11, antiviral12, antioxidant and free radical scavenging activity13-17, cardioprotective18, anti-inflammatory19-20, neuropsychopharmacological21, antiallergic22, radioprotective23, chemopreventive24, larvicidal25, and gastroprotective & antiulcerogenic26 activities.However the reports pertaining to the antioxidant activityofextracts of S. cumini barkis very less. Hence, the present study was designed to screen for various phytochemicals present in the plant and to investigate the in vitro antioxidant potential of various extracts ofS. cumini bark using DPPH, H2O2 and NO free radical scavenging assays.
Materials and Methods
Collection of Plant Material
The barks of S. cumini were collected from the campus of Kalasalingam University, Krishnankoil, Virudhunagar District,Tamil Nadu. The taxonomical identification and authentication was done by Dr. Stephan. M. Sc., Ph. D.,Professor, Department of Botany, The American College, Madurai.A Voucher specimen (AKCP/SCL/07/2016) has been deposited at the Department of Pharmacognosy, ArulmiguKalasalingamCollege of Pharmacy, Srivilliputur for future reference.
Preparation of Plant Extracts
The collected bark was dried under sun shade for 15 days at room temperature and then it was powdered by electrical grinder. The powdered bark of S. cuminihas been percolatedby continuous percolation methodwith Pet. Ether, Hexane, CHCl3, EA, MeOH and water respectively. The general scheme of the extraction protocol is depicted as General scheme 1.
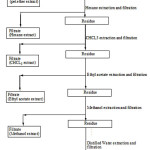 |
Scheme 1: General Scheme of extraction protocol |
Extracts were prepared as per the scheme, extraction of 250g of bark powder in 1.5L of solvent at room temperature for 7 days. The extracts have concentrated by rotary evaporator under reduced presser (SUPERFIT, INDIA) and then lyophilized, the resulting powder of extracts were stored at – 4°Cand used for present study.
Chemical and Reagents
Petroleum ether, Hexane, CHCl3, EA, MeOH, Distilled water, Hydrogen peroxide, Griess reagent, DPPH (2, 2-diphenyl-1-picrylhydrazyl),Hydrochloric acid, Sulphuric acid, Alpha-naphthol, Copper sulphate, Sodium hydroxide, Barfoed’s solution, Benedict’s solution, Potassium mercuric iodide, Potassium bismuth iodide, Iodine, Potassium iodide, Picric acid, Con.HNO3, NH4OH, Millon’s reagent, Ninhydrin, Biuret reagent, Ammonia, 95% Ethanol, Potassium hydroxide, Phenolphthalein, Lead acetate, Ferric chloride, Agar-Agar, Potassium dihydrogen phosphate and Calcium carbonate were obtained from Sigma Aldrich Chemicals and other chemicals and reagents used were of analytical grade.
Phytochemical Screening
The powdered extracts were subjected to Phytochemical investigationto detect for the presence of carbohydrate, protein, fat &oils, steroids, tannins, flavonoids, amino acids and volatile oils as described in literatures. The results were shown as table 1.
Table 1: Phytochemical Screening of various extracts of Syzygium cumini (L.)
| S.No | Phytoconstituents | EXTRACTION | |||||
| Pet. ether | Hexane | CHCL3 | EA | Methanol | Aqueous | ||
| 1 | Carbohydrate | – | – | – | – | – | + |
| 2 | Protein | – | + | – | – | – | + |
| 3 | Amino acid | – | – | – | – | + | – |
| 4 | Steroid | + | – | + | – | – | – |
| 5 | Glycoside | – | + | – | – | – | – |
| 6 | Alkaloid | – | + | + | + | – | + |
| 7 | Tannin | – | – | + | + | + | – |
| 8 | Fat & Oils | + | – | – | – | – | – |
| 9 | Flavonoids | – | – | + | – | + | + |
| 10 | Volatile Oils | – | – | – | – | – | – |
Determination of DPPH Scavenging Activity27
The scavenging reaction between (DPPH) and an antioxidant (H-A) can be written as
(DPPH) + (H-A) → DPPH-H + (A)
(Purple) (Yellow)
Antioxidants react with DPPH, which is a stable free radical and is reduced to the DPPH -H and as consequence the absorbance of DPPH-H decrease compared to DPPH radical. The degree of discoloration indicates the scavenging potential of the antioxidant compounds or extracts in terms of hydrogen donating ability. 1.0 mL of DPPH (0.1 mM) solution was added to 3.0 mL of various extracts in MeOH at different concentration (20-300 μg/mL) and allowed to react in dark room temperature for 30 min. After 30 min, the absorbance was measured at 517 nm. A blank was prepared without adding extract. Ascorbic acid at various concentrations (20 to 300 μg/mL) was used as standard. Lower the absorbance of the reaction mixture indicates higher free radical scavenging activity. The capability to scavenge the DPPH radical was calculated using the following equation:
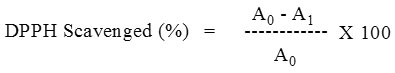
A0 – Absorbance of control, A1 – Absorbance in the presence of plant extract
Determination of Hydrogen Peroxide Scavenging Activity28
A solution of H2O2 (2mM) was prepared in phosphate buffer. Bark extracts at the concentration (20 – 300µg/mL) were added to H2O2 solution (0.6mL) and the total volume was made up to 3mL. The absorbance of the reaction mixture was recorded at 230nm in a spectrophotometer (Shimadzu, UV-1700). A blank solution containing phosphate buffer, without H2O2 was prepared.
The extent of H2O2 scavenging of the plant extracts was calculated as
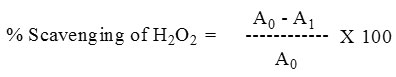
A0 – Absorbance of control, A1 – Absorbance in the presence of plant extract.
Determination of Nitric Oxide Scavenging Activity29
Sodium nitroprusside in Phosphate buffered saline (PBS), at physiological pH, spontaneously generates nitric oxide, which interacts with oxygen to produce nitrite ions that are estimated spectrophotometrically at 546nm.
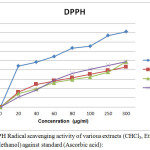 |
Figure 1: DPPH Radical scavenging activity of various extracts (CHCl3, Ethyl acetate and Methanol) against standard (Ascorbic acid): Click here to View figure |
Sodium nitroprusside (10mM) in phosphate buffered saline was mixed with different concentrations (20-300μg/mL) of methanol extract of each plant were dissolved in methanol and incubated at 30°C for 2 hours. The same reaction mixture without the extract but the equivalent amount of ethanol served as the control. After the incubation period, 0.5 ml of Griess reagent (1% sulfanilamide, 2% H3P04 and 0.1% N-(1-naphthyl)-ethylenediaminedihydrochloride) was added. The absorbance of the chromophores that formed during diazotization of the nitrite with sulfanilamide and subsequent coupling with Naphthyl ethylenediamine dihydrochloride was immediately read at 550 nm. Inhibition of nitrite formation by the plant extracts and the standard antioxidant ascorbic acid were calculated relative to the control. Inhibition data (% inhibition) were linearized against the concentrations of each extract and standard antioxidant.
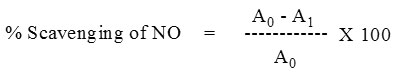
A0 – Absorbance of control, A1 – Absorbance in the presence of plant extract.
Statistical Analysis
All the experiments were performed at least in triplicate using fresh bark sample in each time and all the data points were expressed as Mean ± SEM. Linear regression analysis was used to calculate IC50 for each bark extracts.
Table 2: DPPH Radical scavenging activity of various extracts (CHCl3, Ethyl acetate and Methanol) against standard (Ascorbic acid):
| S.No | Conc.(µg/mL) | Standard | Chloroform | Ethyl acetate | Methanol | ||||
| Absorbance(Mean±SEM) | % Inhibition | Absorbance(Mean±SEM) | % inhibition | Absorbance (Mean±SEM) | %inhibition | Absorbance (Mean±SEM) | %inhibition | ||
| 1 | 20 | 1.059 ±0.0017 | 88.28 | 1.342±0.0016 | 32.15 | 1.359±0.0017 | 29.15 | 1.265 ±0.0015 | 21.58 |
| 2 | 40 | 0.809 ±0.0018 | 96.52 | 1.166 ±0.0019 | 48.71 | 1.199±0.0020 | 39.36 | 1.106 ±0.0017 | 36.62 |
| 3 | 60 | 0.685 ±0.0024 | 109.25 | 0.957 ±0.0021 | 56.16 | 0.997±0.0023 | 52.78 | 0.974±0.0021 | 58.13 |
| 4 | 80 | 0.469 ±0.0027 | 126.72 | 0.803 ±0.0024 | 63.15 | 0.846 ±0.0026 | 59.12 | 0.823 ±0.0025 | 72.52 |
| 5 | 100 | 0.273 ±0.0029 | 131.05 | 0.643 ±0.0028 | 70.65 | 0.683 ±0.0029 | 64.69 | 0.730 ±0.0028 | 81.34 |
| 6 | 250 | 0.243 ±0.0031 | 154.05 | 0.592 ±0.0030 | 78.32 | 0.637±0.0031 | 75.06 | 0.656±0.0032 | 89.75 |
| 7 | 300 | 0.239 ±0.0033 | 162.34 | 0.453 ±0.0034 | 86.19 | 0.542±0.0035 | 97.58 | 0.582±0.0036 | 97.86 |
| IC50 | IC50 = 6.1 µg/mL | IC50 = 41 µg/mL | IC50 = 57 µg/mL | IC50 = 53 µg/mL | |||||
Results and Discussion
The preliminary phytochemical analysis of S. cumini (L) bark extracts showed the presence of various phytochemical constituents such as steroids, alkaloids, tannins and flavonoids in which tannins were present in all three extracts. Glycosides, volatile oil and fat & oil were absent in most of the bark extracts. The herbal research studies revealed that tannins and flavonoids are responsible for antioxidant activity in order to treat neuro-degenerative disorders, CVS diseases, diabetes and treatment of cancer.In DPPH radical scavenging assay, the compound’s ability to reduce the DPPH (a stable free radical). DPPH is nitrogen-centered stable free radical having a maximum absorbance at 517 nm in methanolic solution. It becomes a stable diamagnetic molecule on accepting an electron or hydrogen atom. In the presence of an extract capable of donating a hydrogen atom, the free radical nature of DPPH is lost and the purple colour change to yellow (diphenyl picrylhydrazine). The scavenging ability results were showed that absorbance value with 1.342 ± 0.0016, 1.359 ± 0.0017 and 1.265 ± 0.0015 (Mean ± SEM) for CHCl3, EA and MeOH extracts respectively at minimum concentration of 20 µg/mL and maximum concentration of 300 µg/mL about 0.453 ± 0.0034, 0.542 ± 0.0035 and 0.582 ± 0.0036 (Mean±SEM) respectively. The IC50 value of CHCl3, EA and MeOH extracts were found to be 41, 57 and 53 µg/mL respectively. The scavenging abilityas % inhibition of the S. cumini (L) bark extracts of CHCl3, EA and MeOH by DPPH method has clearly showed a dose-dependent antioxidant activity.
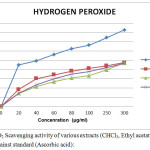 |
Figure 2: H2O2 Scavenging activity of various extracts (CHCl3, Ethyl acetate and Methanol) against standard (Ascorbic acid):
|
Table 3: H2O2 scavenging activity of various extracts (CHCl3, Ethyl acetate and Methanol) against standard (Ascorbic acid):
| S.No | Conc.(µg/mL) | Standard | Chloroform | Ethyl acetate | Methanol | ||||
| AbsorbanceMean±SEM | %Inhibition | AbsorbanceMean±SEM | % inhibition | AbsorbanceMean±SEM | % inhibition | AbsorbanceMean±SEM | %Inhibition | ||
| 1 | 20 | 1.059±0.0017 | 89.58 | 1.490±0.0018 | 36.58 | 1.371±0.0016 | 27.95 | 1.377 ±0.0017 | 28.54 |
| 2 | 40 | 0.809±0.0018 | 98.35 | 1.283±0.0020 | 59.78 | 1.201±0.0019 | 42.56 | 1.214 ±0.0020 | 46.82 |
| 3 | 60 | 0.785±0.0021 | 112.01 | 1.087±0.0023 | 68.05 | 1.056±0.0021 | 53.69 | 1.071±0.0023 | 59.51 |
| 4 | 80 | 0.682±0.0024 | 124.73 | 0.901±0.0027 | 76.25 | 0.892±0.0025 | 61.78 | 0.925 ±0.0026 | 69.42 |
| 5 | 100 | 0.563±0.0027 | 132.24 | 0.842±0.0030 | 82.32 | 0.806±0.0029 | 66.56 | 0.830 ±0.0029 | 75.36 |
| 6 | 250 | 0.421±0.0030 | 148.62 | 0.765±0.0032 | 88.09 | 0.756±0.0031 | 78.92 | 0.732±0.0032 | 83.78 |
| 7 | 300 | 0.395±0.0034 | 165.36 | 0.623±0.0036 | 94.61 | 0.683±0.0033 | 94.36 | 0.612±0.0036 | 92.75 |
| IC50 | IC50 =5.5 µg/ml | IC50 =31 µg/ml | IC50 =46 µg/ml | IC50 =44 µg/ml | |||||
To further prove the antioxidant activity, the antioxidant scavenging activity of the selected extracts of S.cumini was carried out by hydroxyl radical scavenging assay. The Fenton reaction generates OH* radicals which degrade DNA using Fe2+ salts as an important catalytic component and may cause to DNA fragmentation and DNA strand breakage. The results of antioxidant scavenging assay showed absorbance value of 1.490 ± 0.0018, 1.371 ± 0.0016 and 1.37 ± 0.0017 (Mean±SEM) for CHCl3, EA and MeOH extracts at minimum concentration of 20 µg/mL respectively and 0.623 ± 0.0036, 0.683 ± 0.0033 and 0.612 ± 0.0036 (Mean ± SEM) for CHCl3, EA and MeOH extracts at maximum concentration of 300 µg/mL respectively. Moreover, the IC50 value of CHCl3, EA and MeOH extracts of S. cumini (L) bark was found to be 31, 46 and 44 µg/mL respectively.
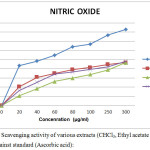 |
Figure 3: No Scavenging activity of various extracts (CHCl3, Ethyl acetate and Methanol) against standard (Ascorbic acid): |
Table 4: NO scavenging activity of various extracts (CHCl3, Ethyl acetate and Methanol) against standard (Ascorbic acid):
| S.No | Conc.(µg/mL) | Standard | Chloroform | Ethyl acetate | Methanol | ||||
| AbsorbanceMean±SEM | %Inhibition | AbsorbanceMean±SEM | % inhibition | AbsorbanceMean±SEM | % inhibition | AbsorbanceMean±SEM | %Inhibition | ||
| 1 | 20 | 1.063 ±0.0017 | 87.26 | 1.160±0.0015 | 41.32 | 1.192±0.0014 | 21.52 | 1.120±0.0012 | 32.96 |
| 2 | 40 | 0.819 ±0.0018 | 96.37 | 1.118±0.0021 | 61.01 | 1.143±0.0017 | 36.56 | 1.014 ±0.0015 | 50.51 |
| 3 | 60 | 0.735±0.0021 | 110.01 | 1.005±0.0024 | 69.74 | 1.017±0.0018 | 52.74 | 0.913±0.0018 | 68.52 |
| 4 | 80 | 0.698±0.0024 | 128.23 | 0.921±0.0027 | 78.05 | 0.939±0.0020 | 60.04 | 0.839±0.0021 | 73.02 |
| 5 | 100 | 0.525 ±0.0027 | 133.54 | 0.855±0.0029 | 83.12 | 0.878±0.0024 | 67.58 | 0.755 ±0.0024 | 78.51 |
| 6 | 250 | 0.465±0.0030 | 153.73 | 0.739 ±0.0032 | 89.45 | 0.754±0.0028 | 77.45 | 0.721±0.0029 | 84.23 |
| 7 | 300 | 0.336±0.0034 | 165.78 | 0.613±0.0036 | 93.78 | 0.624±0.0031 | 93.71 | 0.654±0.0034 | 96.27 |
| IC50 | IC50 =6.8 µg/ml | IC50 =30 µg/ml | IC50 =58 µg/ml | IC50 =39 µg/ml | |||||
Nitric oxide (NO) is one of the strong pleiotropic mediators in physiological processes as well as pathological conditions. Formation of peroxynitrite (ONOO–) as a strong oxidant is responsible for oxidative damage of proteins in living systems. Hence, we also evaluated the extracts antioxidant activity using NO scavenging assay. The results of NO scavenging activity showed an absorbance value of 1.160 ± 0.0015, 1.192 ± 0.0014 and 1.120 ± 0.0012 (Mean±SEM) for CHCl3, EA and MeOH extracts at minimum concentration of 20 µg/mL respectively and0.613 ± 0.0036, 0.624 ± 0.0031 and 0.654 ± 0.0034 (Mean ± SEM) respectively at a maximum concentration of 300 µg/mL. The IC50 value of CHCl3, EA and MeOH extracts of S. cumini (L) bark was found to be 30, 58 and 39 µg/mL respectively. The extracts showed similar IC50 value irrespective of the solvents used for extraction. Since, tannins were present in all the extracts and it showed similar IC50 values irrespective of the solvents used, it can be concluded that tannins present in S. cumini may be the reason for its antioxidant property which can be further studied.
Conclusion
Results from current research revealed that CHCl3, EA and MeOH extracts of S. cumini possessed good antioxidant activity using DPPH, H2O2 and NO scavenging assay. The preliminary phytochemical screening of that indicated the presence of tannins in all the three extracts. Based on the antioxidant assay data, it could be concluded that tannins present in S. cumini may be the reason for its antioxidant activity which has to be studied further. Also extracts of S. cumini can be further characterized and studied in detail for its mechanism of action in order to develop harmless, cost-effective and targeted antioxidant agent for the benefit of mankind.
References
- Khanna, R. D.; Karki, K.; Pande, D.; Negi, R.; Khanna, R. S.Interdiscip J Microinflammation.2014, 1, 1-5.
- Nemat, K.; Yadollah, S.; Mahdi, M.Recent Patents on Inflammation & Allergy Drug Discovery. 2009, 3, 73-80.
CrossRef - Gill, R.; Tsung, A.; Billiar, T. Free Radic. Biol. Med.2010, 48, 1121-1132.
CrossRef - Schetter, A. J.; Heegaard, N. H.; Harris, C. C.Carcinogenesis.2010, 31, 37-49.
CrossRef - Gilmore, T. D. Oncogene. 2006, 25, 6680-6684.
CrossRef - Ayyanar, M.; Subash-Babu, P.Asian Pac. J. Trop. Biomed.2012, 2, 240-246.
CrossRef - Shafi, P. M.;Rosamma, M. K.; Kaiser, J.; Reddy, P.S.Fitoterapia.2002, 73, 414–416.
CrossRef - Gowri, S. S.; Vasantha, K. Inter.J. Pharm Tech Res.2010, 2, 1569-1573.
- Hofling, J. F.;Anibal, P. C.; Obandopereda, G. A.;Peixoto, I. A. T.;Furletti, V. F.;Foglio, M. A.; Gonçalve, R. B.Braz. J. Bio.2010, 70, 1065-1068.
CrossRef - Meshram,G. A.;Yadav, S. S.;Shinde, D.;Patil, B.;Singh, D.J. Pharma. Sci & Res.2011, 3, 1060-1063.
- Satish, S.;Mohana, D. C.;Raghavendra, M. P.;Raveesha, K. A.Inter. J. Agri. Tech.2007, 3, 109-119.
- Sood, R.; Swarup, D.; Bhatia, S.;Kulkarni, D. D.; Dey, S.; Saini, M.; Dubey, S. C.Ind.J. Exp.Bio.2012, 50,179-186.
- Banerjee,A.; Dasgupta, N.; Bratati, D. Food Chemistry.2005, 90, 727-733.
CrossRef - Ruan, Z. P.; Zhang, L. L.; Yi Ming, L.Molecules.2008, 13,1420-3049.
- Bajpai, M.;Pande, A.; Tewari, S. K.; Prakash, D. Inter. J. Food. Sci and Nutri.2005, 56,287-291.
CrossRef - Hasan, S. M. R.;Md, Hossain. M. R.; Akter, J. M.; Md,Mazumder, E. H.; Rahman, S.J Medi Plant Res.2009, 3,875-879.
- Faria, A.;F,Marques.; M. C.; Mercadante, A. Z.Food Chemistry.2011, 126, 1571-1578.
CrossRef - Mastan, S. K.; Chaitanya, G.; Bhavya, L.T.; Srikanth, A.; Sumalatha, G.; Eswar, K. K.Der Pharmacia Lettre.2009, 1, 143-149.
- Muruganandan, S.; Srinivasan, K.; Chandra, S.;Tandan, S. K.;Lal, J.; Raviprakash, V.Fitoterapia.2001, 72, 369-375.
CrossRef - Kumar, A.; Ilavarasan, R.; Jayachandran, T.; Deecaraman, M.; Kumar, R. M.; Aravindan, P.; Padmanabhan, N.;Krishan, M.R. V. Afr. J. Biotechnol.2008, 7, 941-943.
- Kumar, A.;Padmanabhan, N.; Krishnan, M.R.V.Pak. J. Nutri.2007, 6, 698-700.
CrossRef - Brito, F. A.;Lima, L. A.; Ramos, M.F. S.; Nakamura, M. J.; Cavalher-Machado, S. C.; Siani, A. C.; Henriques, G.M. O.; Sampaio, A. L. F.Braz. J. Med. Bio. Res. 2007, 40, 105-115.
CrossRef - Jagetia, G. C.;Baliga,M. S.Toxicol. Lett.2002, 132, 19-25.
CrossRef - Parmar, J.; Sharma, P.; Verma, P.; Goyal. P. K. Asian Pac. J. Cancer Prevent.2010, 11, 261-265.
CrossRef - Raghavendra, B. S.; Prathiba, K. P.;Vijiyan, V. A.;J. Entamol.2011, 8, 491-496.
- Ramirez.Clinical Hemorheology and Microcirculation.2003, 29, 253-261.
- Brand, W. W.; Cuvelier, M. E.; Berset, C.Lebensmittel-Wissenschaft und-Technologie/Food Science and Technology.1995, 28, 25-30.
- Fan, Y. M.; Xu, L. Z.; Gao, J.; Wang, Y.; Tang, X. H.; Zhao, X. N.Fitoterapia.2004, 75, 253-260.
CrossRef - Gulcin, I.; Alici, H. A.; Cesur, M. Chem. Pharm. Bull.2005, 53, 281-285.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









