Antibacterial Activity Exerted by Some Diazabicyclo-Steroid Derivatives Against Staphylococcus Aureus and Streptococcus Pneumoniae
Hau-Heredia Lenin1, Figueroa-Valverde Lauro1, García-Cervera Elodia1, López-Ramos Maria1, Díaz-Cedillo Francisco2, Pool-Gómez Eduardo1, Rosas-Nexticapa Marcela3, Herrera-Meza Socorro4 and Cauich-Carrillo Regina1
1Laboratory of Pharmaco-Chemistry, Faculty of Chemical Biological Sciences, University, University Autonomous of Campeche, Av. Agustín Melgar s/n, Col Buenavista C.P. 24039 Campeche, Camp.,México.
2Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional. Prol. Carpio y Plan de Ayala s/n Col. Santo Tomas, México, D.F. C.P. 11340.
3Facultad de Nutrición, Universidad Veracruzana, Médicos y Odontologos s/n C.P. 91010, Unidad del Bosque Xalapa Veracruz, México.
4Instituto de Investigaciones Psicológicas, Universidad Veracruzana. Av. Dr. Luis Castelazo Ayala s/n Col Industrial Animas. C.P. 91190, Xalapa, Veracruz, México.
Corresponding Author E-mail: Lauro1999@yahoo
DOI : http://dx.doi.org/10.13005/ojc/330564
The aim of this study was synthesize three diazabicyclo-steroid derivatives to evaluate its antibacterial activity. This process involved a series of reactions such as; i) cycloaddition [2 + 2] of 5-hexyn-1-ol to OTBS-testosterone (1) or progesterone (2) or OTBS-pregnenolone (3) to form cyclobutene-ol-steroid derivatives (4 or 5 or 6); ii) the compounds 4 or 5 or 6 were reacted with ethylenediamine to form steroid-amino conjugates (7 or 8 or 9); iii) alkynylation of 7 or 8 or 9 with 5-hexyn-1-ol to form the steroid-amino-hexynol conjugates (10 or 11 or 12); iv) preparation of the cyclobuta-ynal-steroid derivatives (13 or 14 or 15) by the reaction of 10 or 11 or 12 with DMSO; v) amination of 13 or 14 or 15 with ethylenediamine to form new amino-steroid derivatives 16 or 19 or 21; vi) removal of the tert-butyldimethylsilyl from 16 or 21 with hydrofluoric acid to form hydroxyl-steroids (17 and 22); vii) preparation of 1,4-diazacycloundeca-5,11-dien-steroid derivatives by the reaction 17 or 19 or 22 with Copper(II) chloride. In order to evaluate the possibility of that compounds synthesized may have biological activity; in this study its antibacterial effect on Streptococcus pneumoniae and Staphylococcus aureus bacteria was evaluated. The results indicate that compound 20 exert higher antibacterial activity against Staphylococcus aureus and Streptococcus pneumoniae compared with 18 and 23 via interaction DNa-gyrase. In conclusion, these data indicate that antibacterial activity exerted by the compounds 20 depend of their structure chemical in comparison with the other steroid derivatives involved in this study.
KEYWORDS:Testosterone; Pregnenolone; Progesterone Steroid; Derivatives; Antibacterial
Download this article as:| Copy the following to cite this article: Lenin H. H, Lauro F. V, Elodia G. C, Maria L. R, Francisco D. C, Eduardo P. G, Marcela R. N, Socorro H. M, Regina C. C. Antibacterial Activity Exerted by Some Diazabicyclo-Steroid Derivatives Against Staphylococcus Aureus and Streptococcus Pneumoniae. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Lenin H. H, Lauro F. V, Elodia G. C, Maria L. R, Francisco D. C, Eduardo P. G, Marcela R. N, Socorro H. M, Regina C. C. Antibacterial Activity Exerted by Some Diazabicyclo-Steroid Derivatives Against Staphylococcus Aureus and Streptococcus Pneumoniae. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=39232 |
Introduction
Infectious diseases are one of the main causes of morbidity-mortality in the world1,2. There are several reports which indicate that some causal agents,such as Staphylococcus aureus3, Streptococcus pneumoniae4 and othersare involved in infection diseases developed. Although there are many therapeutic agents for treatment of these bacterial microorganisms5, 6, unfortunately prolonged antibiotic therapy induces bacterial-resistance, because some bacteriahave developed ways to circumvent the effects of antibiotics7, 8. Therefore, antibiotic resistance to bacteria can be considered a serious threat for the human health; this fact requires an international approach to its management. In this sense, new drugs have been developed for control of bacterial resistance; for example, the preparation of diazabicycle derivatives as antibacterial agents against Staphylococcus aureus strains9 Other data indicate the synthesis and antibacterial activity of some 1,3-diazabicyclo-carbapen derivatives 2,4-dichloro-5-fluorophenyl derivatives against Staphylococcus aureus strain10. In addition, some 3,7-diazabicyclo[3.3.1]nonane azines were prepared as antibacterial agents against Staphylococcus aureus11. In addition a study showed the synthesis of 9-alkyl-l,5-diazabicyclo[4.3.0]non-5-enes which decreased the bacterial growth of Staphylococcus aureus12. Also, other report showed the preparation of 1,4-diazabicyclo[2.2.2]octane with antibacterial activity against a Staphylococcus aureus strain13. Additionaly, other report shown that the diazabycycle derivative (1,3-diazaadamante) exerted antibacterial activity against Staphylococcus aureus14.
On the other hand, a 3,7-diazabicyclo[3.3.1]nonane derivative was synthesized as antibacterial agent against Streptococcus pneumoniae15, 16. Additionally, other data showed the synthesis of the compound 3,8-Diazahicyclo[3.2.l]octane17 which inhibits the bacterial growth of Streptococcus pneumoniae18, 19. All these experimental results show that several diazabicyclo derivatives can induce antibacterial effects against Staphylococcus aureus and Streptococcus pneumoniae. Analyzing these data, in this study three diazabicyclo-steroid derivatives were synthesized and their antibacterial activity against Staphylococcus aureus and Streptococcus pneumoniae was evaluated in vitro.
Experimental
General Methods
The testosterone derivative and pregnenolone (OTBS-Testosterone and OTBDS-pregnenolone) were prepared using methods previously reported20. The other reagents used in this study were purchased from Sigma-Aldrich Co. Ltd. The melting point was determined on an Electrothermal (900 model). 1H and 13C NMR spectra were recorded on a Varian VXR-300/5 FT NMR spectrometer at 300 and 75.4 MHz in CDCl3 using TMS as internal standard. EIMS spectra were obtained with a Finnigan Trace GCPolaris Q. spectrometer. Elementary analysis data were acquired from a Perkin Elmer Ser. II CHNS/0 2400 elemental analyzer.
Preparation of Cyclobuta-3-One-Steroid Derivatives
A solution of 1 or 2 or 3 (0.50 mmol), 5-hexyn-1-ol (60 µl, 0.54 mmol), Iron(III) chloride anhydrous (80 mg, 0.49 mmol) in 5 ml of methanol was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water (4:1).
8-(tert-Butyl-dimethyl-silanyloxy)-2-(4-Hydroxy-butyl)-5a,7a-dimethyl-4,5,5a,5b,6,7,7a,8,9,10,10a,10b, 11,12-tetradecahydro-2aH-cyclobuta[j]cyclopenta[a]phenanthren-3-one (4)
yielding 67 % of product, m.p. 74-76 oC; IR (Vmax, cm-1): 3400, 1720 and 1058; 1H NMR (300 MHz, CDCl3) δH: 0.08 (s, 6H), 0.68 (s, 3H), 0.86 (s, 9H), 0.94-1.04 (m, 2H), 1.08 (s, 3H), 1.20-1.48 (6H), 152-1.60 (m, 4H), 1.62-2.10 (m, 8H), 2.20 (m, 2H), 2.22-3.54 (m, 5H), 3.60 (broad, 1H), 3.66 (m, 2H), 5.70 (d, 1H, J = 1.82 Hz) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.60 (C-24, C-31), 11.32 (C-21), 14.80 (C-20), 18.02 (C-32), 21.34 (C-18), 23.52 (C-8), 24.30 (C-27), 25.24 (C-11), 25.50 (C-33, C-34, C-35), 31.06 (C-7), 31.82 (C-10), 32.44 (C-12), 32.54 (C-28), 36.54 (C-3), 36.70 (C-19), 37.02 (C-26), 38.50 (C-13), 40.40 (C-9), 42.24 (C-1), 43.30 (C-5), 49.78 (C-2), 52.00 (C-4), 60.16 (C-15), 62.55 (C-29), 81.62 (C-6), 134.52 (C-17), 147.68 (C-16), 210.62 (C-14) ppm. EI-MS m/z: 500.36 Anal. Calcd. for C31H52O3Si: C, 74.34; H, 10.47; O, 9.58; Si, 5.61. Found: C, 74.26; H, 10.36.
8-Acetyl-2-(4-hydroxy-butyl)-5a,7a-dimethyl-4,5,5a,5b,6,7,7a,8,9,10,10a,10b,11,12-tetradecahydro-2aH-cyclobuta[j]cyclopenta[a]phenanthren-3-one (5)
yielding 44 % of product, m.p. 80-82 oC; IR (Vmax, cm-1): 3404 and 1722; 1H NMR (300 MHz, CDCl3) δH: 0.70 (s, 3H), 1.06 (s, 3H), 1.20-1.46 (8H), 1.50-1.58 (m, 4H), 1.66-2.10 (m, 8H), 2.12 (s, 3H), 2.20 (t, 2H, J = 13.20 Hz), 2.22-3.00 (m, 5H), 3.60 (broad, 1H), 3.66 (t, 2H, J = 11.00 Hz), 5.70 (d, 1H, J = 1.32 Hz) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 13.40 (C-21), 14.80 (C-20), 21.52 (C-7), 23.02 (C-18), 24.30 (C-24), 24.44 (C-17), 25.24 (C-10), 30.74 (C-30), 31.82 (C-9), 32.44 (C-11), 32.54 (C-25), 35.64 (C-3), 37.02 (C-23), 38.48 (C-12), 39.02 (C-6), 40.40 (C-8), 42.24 (C-1), 44.28 (C-5), 51.19 (C-2), 57.72 (C-4), 60.16 (C-14), 62.55 (C-26), 63.98 (C-19), 134.52 (C-16), 147.68 (C-15), 208.28 (C-28), 210.62 (C-13) ppm. EI-MS m/z: 412.29 Anal. Calcd. for C27H40O3: C, 78.60; H, 9.77; O, 11.63. Found: C, 78.52; H, 9.68.
1-[8-(tert-Butyl-dimethyl-silanyloxy)-10-(4-hydroxy-butyl)-3a,3b-dimethyl-1,2,3,3a,4,5,5a,5b,6,7,11a, 12,12a,12b-hexadecahydro-cyclobuta[k]cyclopenta[a]phenanthren-3-yl] ethanonone (6)
yielding 56 % of product, m.p. 148-150oC; IR (Vmax, cm-1): 3402, 1720 and 1058; 1H NMR (300 MHz, CDCl3) δH: 0.06 (s, 6H), 0.70 (s, 3H), 0.90 (s, 9H), 0.98 (s, 3H), 1.14-1.46 (m, 9H), 1.50 (m, 2H), 1.52 (m, 1H), 1.56-2.00 (m, 7H), 2.02 (m, 2H), 2.12 (s, 3H), 2.16-2.20 (m, 3H), 2.58-3.50 (m 5H), 3.60 (broad, 1H), 3.66 (t, 2H, J = 11.00 Hz), 5.40 (d, 1H, J = 1.32 Hz) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.60 (C-22, C-32), 13.70 (C-24), 16.90 (C-24), 18.50 (C-33), 21.22 (C-10), 24.12 (C-15), 24.20 (C-16), 24.90 (C-28), 26.10 (C-34, C-36, C-37), 28.34 (C-35), 28.52 (C-2), 32.54 (C-29), 32.88 (C-6), 33.34 (C-14), 35.00 (C-3), 35.14 (C-11), 35.62 (C-27), 38.68 (C-4), 39.02 (C-9), 43.20 (C-13), 44.14 (C-8), 44.80 (C-5), 51.12 (C-12), 56.42 (C-7), 62.55 (C-30), 63.78 (C-17), 71.56 (C-1), 133.92 (C-19), 153.90 (C-18), 208.58 (C-25) ppm. EI-MS m/z: 528.39 Anal. Calcd. for C33H56O3Si: C, 74.94; H, 10.67; O, 9.08; Si, 5.31. Found: C, 74.83; H, 10.54.
Preparation of Cyclobutene-1-Ol-Steroid-Amino Conjugates
A solution of 4 or 5 or 6 (0.50 mmol), ethylenediamine (60 µl, 0.90 mmol) and boric acid (50 mg, 0.80 mmol), in 5 ml of methanol was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water (3:1).
4[3-(2-Amino-ethylimino)-8-(tert-butyl-dimethyl-silanyloxy)-5a,7a-dimethyl-2a,3,4,5,5a,5b,6,7,7a,8, 9,10,10a,10b,11,12-hexadecahydro-cyclobuta[j]cyclopenta[a]phenan- thren-2yl]-butan-1-ol (7)
yielding 55 % of product, m.p. 120-122oC; IR (Vmax, cm-1): 3402, 3370 and 1058; 1H NMR (300 MHz, CDCl3) δH: 0.08 (s, 6H), 0.68 (s, 3H), 0.88 (s, 9H), 0.94 (m, 1H), 0.98 (s, 3H), 1.06-1.48 (8H), 1.50 (m, 2H), 1.56-1.60 (m, 2H), 1.62 (m, 2H), 1.64-1.88 (m, 5H), 2.15 (t, 2H, J = 13.20 Hz), 2.22-2.42 (m, 3H), 3.10-3.50 (m, 4H), 3.54 (m, 1H), 3.66 (t, 2H, J = 11.00 Hz), 4.10 (broad, 3H), 5.20 (m, 1H), 5.40 (d, 1H, J = 1.32 Hz) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.60 (C-24, C-34), 11.34 (C-21), 15.60 (C-20), 18.02 (C-35), 21.32 (C-18), 23.52 (C-8), 24.38 (C-30), 25.14 (C-11), 25.50 (C-36, C-37, C-38), 26.64 (C-12), 27.22 (C-13), 31.04 (C-7), 32.02 (C-10), 32.54 (C-31), 36.50 (C-29), 36.54 (C-3), 36.70 (C-19), 41.00 (C-27), 42.04 (C-9), 43.30 (C-5), 45.16 (C-1), 47.40 (C-2), 49.08 (C-15), 52.00 (C-4), 53.60 (C-26), 62.55 (C-32), 81.70 (C-6), 132.34 (C-17), 150.38 (C-16), 169.34 (C-14) ppm. EI-MS m/z: 542.42 Anal. Calcd. for C33H58N2O2Si: C, 73.01; H, 10.77; N, 5.16; O, 5.89; Si, 5.17. Found: C, 73.00; H, 10.68.
4-{3-(2-Amino-ethylimino)-8-[1-(2-amino-ethylimino)-ethyl]-5a,7a-dimethyl-2a,3,4,5,5a,5b,6,7,7a,8,9, 10,10a,10b,11,12-hexadecahydro-cyclobuta[j]cyclopenta[a]phenan- thren-2-yl}-butan-1-ol (8)
yielding 64 % of product, m.p. 70-72oC; IR (Vmax, cm-1): 3402 and 3332; 1H NMR (300 MHz, CDCl3) δH: 0.88 (s, 3H), 0.98 (s, 3H), 1.20-1.44 (m, 7H), 1.50 (m, 2H), 1.52-1.58 (m, 3H), 1.62 (m, 2H), 1.76 (m, 1H), 1.80 (s, 3H), 1.82-2.12 (m, 4H), 2.18 (t, 2H, J = 13.20 Hz), 2.22-2.40 (m, 5H), 3.09 (m, 2H), 3.10-3.50 (m, 4H), 3.52 (m, 2H), 3.66 (t, 2H, J = 11.00 Hz), 4.20 (broad, 5H), 5.20 (m, 1H), 5.40 (d, 1H, J = 1.82 Hz) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 13.20 (C-21), 15.60 (C-20), 16.70 (C-36), 21.92 (C-7), 24.40 (C-27), 25.18 (C-10), 25.28 (C-17), 26.40 (C-18), 26.62 (C-11), 27.28 (C-12), 32.04 (C-9), 32.54 (C-28), 36.52 (C-26), 36.56 (C-3), 38.28 (C-6), 41.00 (C-24), 41.02 (C-34), 42.04 (C-8), 42.82 (C-5), 45.17 (C-1), 48.80 (C-2), 49.06 (C-14), 53.10 (C-33), 53.60 (C-23), 56.22 (C-4), 62.55 (C-29), 63.08 (C-19), 132.32 (C-16), 150.38 (C-15), 156.78 (C-31), 169.28 (C-13) ppm. EI-MS m/z 496.41 Anal. Calcd. for C31H52N4O: C, 74.95; H, 10.55; N, 11.28; O, 3.22. Found: C, 74.84; H, 10.46.
4-[3-[1-(2-Amino-ethylimino)-ethyl]-8-(isopropyl-dimethyl-silanyloxy)-3a,5b-dimethyl-1,2,3,3a,4,5,5a, 5b,6,7,8,9,11a,12,12a,12b-hexadecahydro-cyclobuta[k]cyclopenta [a]phenanthren- 10-yl]-butan-1-ol (9)
yielding 45 % of product, m.p. 258-260oC; IR (Vmax, cm-1): 3370, 3330 and 1060; 1H NMR (300 MHz, CDCl3) δH: 0.07 (s, 6H), 0.88 (s, 3H), 0.90 (s, 9H), 0.98 (s, 3H), 1.14-1.40 (m, 7H), 1.50 (m, 2H), 1.51 (m, 1H), 1.52 (m, 2H), 1.53-1.78 (m, 6H), 1.80 (s, 3H), 1.82-1.94 (m, 2H), 2.00 (m, 2H), 2.12-2.40 (m, 5H), 3.08-3.52 (m, 4H), 3.53 (m, 1H), 3.66 (t, 2H, J = 11.00 Hz), 4.10 (broad, 3H), 5.40 (d, 1H, J = 1.82 Hz) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.60 (C-22, C-35), 13.20 (C-24), 16.70 (C-38), 16.90 (C-23), 18.44 (C-36), 21.68 (C-10), 24.90 (C-31), 25.22 (C-15), 26.04 (C-37, C-39, C-40), 26.40 (C-16), 28.52 (C-2), 32.54 (C-32), 33.30 (C-14), 33.80 (C-6), 35.00 (C-3), 35.14 (C-11), 35.57 (C-30), 38.28 (C-9), 38.60 (C-4), 41.00 (C-28), 42.82 (C-8), 43.15 (C-13), 44.80 (C-5), 51.14 (C-12), 53.10 (C-27), 57.36 (C-7), 62.55 (C-33), 63.08 (C-17), 71.56 (C-1), 133.94 (C-19), 153.88 (C-18), 156.70 (C-25) ppm. EI-MS m/z 570.45 Anal. Calcd. for C35H62N2O2Si: C, 73.63; H, 10.95; N, 4.91; O, 5.60; Si, 4.92. Found: C, 73.52; H, 10.83.
Alkynylation of Amino Groups
A solution of 7 or 8 or 9 (0.50 mmol), 5-hexyn-1-ol (130 µl, 1.18 mmol) and cupric chloride (120 mg, 0.89 mmol), in 5 ml of methanol was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from hexane:methanol:water (1:4:2).
6-{2-[8-tert-Butyl-dimethyl-silanyloxy)-2-(3-hydroxy-propyl)-5a,7a-dimethyl-4,5,5a,5b,6,7,7a,8,9,10, 10a,10b,11,12-tetradecahydro-2aH-cyclobuta[j]cyclopenta[a]phenanthren-3-ylideneamino]-ethylami no}-hex-5-yn-1-ol (10)
yielding 38 % of product, m.p. 130-132 oC; IR (Vmax, cm-1): 3400 and 1058; 1H NMR (300 MHz, CDCl3) δH: 0.06 (s, 6H), 0.68 (s, 3H), 0.88 (s, 9H), 0.90 (m, 1H), 0.98 (s, 3H), 1.04-1.56 (9H), 1.58 (m, 2H), 1.60-1.62 (m, 2H), 1.64 (m, 2H), 1.68 (m, 2H), 1.78-2.22 (m, 6H), 2.26 (m, 2H), 2.30 (m, 2H), 2.40 (m, 1H), 3.20 (t, 2H, J = 13.60 Hz), 3.52 (m, 2H), 3.53 (m, 1H), 3.56 (m, 2H), 3.66 (t, 2H, J = 11.00 Hz), 3.80 (broad, 3H), 5.40 (d, 1H, J = 1.32 Hz), 5.60 (m, 1H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.60 (C-22, C-40), 11.40 (C-23), 15.60 (C-24), 16.18 (C-31), 17.80 (C-41), 21.32 (C-13), 23.14 (C-37), 23.54 (C-11), 25.14 (C-9), 25.72 (C-42, C-43, C-44), 25.80 (C-32), 26.64 (C-14), 27.22 (C-15), 30.06 (C-33), 31.04 (C-10), 32.02 (C-8), 33.40 (C-36), 36.50 (C-4), 36.72 (C-12), 42.04 (C-7), 43.30 (C-2), 45.16 (C-6), 47.40 (C-5), 47.68 (C-17), 51.72 (C-26), 51.98 (C-3), 54.14 (C-27), 61.80 (C-38), 62.05 (C-34), 78.44 (C-30), 81.66 (C-1), 87.30 (C-29), 133.52 (C-18), 150.58 (C-16), 169.34 (C-16) ppm. EI-MS m/z: 624.46 Anal. Calcd. for C38H64N2O3Si: C, 73.02; H, 10.32; N, 4.48; O, 7.68; Si, 4.49. Found: C, 73.00; H, 10.26.
6-{2-[8-{1-[2-(6-Hydroxy-hex-1-ynylamino)-ethylimino]-ethyl}-2-(3-hydroxy-propyl)-5a,7a-dimethyl-4,5,5a,5b,6,7,7a,8,9,10,10a,10b,11,12-tetradecahydro-2aH-cyclobuta[j]cyclopenta[a]phenanthren-3-ylideneamino]-ethylamino}-hex-5-yn-1-ol (11)
yielding 64 % of product, m.p. 133-134oC; IR (Vmax, cm-1): 3402 and 3330; 1H NMR (300 MHz, CDCl3) δH: 0.88 (s, 3H), 0.98 (s, 3H), 1.20-1.56 (m, 10H), 1.58 (m, 4H), 1.66 (m, 4H), 1.68 (s, 3H), 1.69 (m, 2H), 1.78-2.22 (m, 7H), 2.26 (t, 2H, J = 13.20 Hz), 2.28 (m, 1H), 2.30 (m, 4H), 2.38-2.40 (m, 2H) 3.16 (m, 4H), 3.52 (t, 2H, J = 11.00 Hz), 3.56 (m, 4H), 3.64 (m, 4H), 3.70 (broad, 5H), 5.40 (d, 1H, J = 1.82 Hz), 5.60 (m, 1H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 13.20 (C-20), 15.60 (C-21), 16.20 (C-29, C-40), 21.92 (C-10), 22.60 (C-49), 23.20 (C-46), 25.18 (C-8), 25.80 (C-30, C-41), 26.40 (C-12), 26.62 (C-13), 26.64 (C-14), 27.28 (C-15), 30.02 (C-31, C-42), 32.04 (C-7), 33.42 (C-45), 36.52 (C-3), 38.18 (C-9), 42.06 (C-8), 42.82 (C-1), 45.17 (C-5), 47.68 (C-17), 48.80 (C-4), 51.22 (C-24), 51.76 (C-35), 52.44 (C-2), 54.10 (C-25, C-36), 56.70 (C-11), 61.80 (C-47), 62.05 (C-32, C-43), 78.48 (C-28, C-39), 87.28 (C-27, C-38), 133.52 (C-19), 150.58 (C-18), 162.68 (C-22), 169.28 (C-16) ppm. EI-MS m/z 674.51 Anal. Calcd. for C42H66N4O3: C, 74.73; H, 9.86; N, 8.30; O, 7.11. Found: C, 74.64; H, 9.78.
6-(2-{1-[8-(tert-Butyl-dimethyl-silanyloxy)-10-(4-hydroxy-butyl)-3a,5b-dimethyl-1,2,3, 3a,4,5,5a,5b, 6,7,8,9,11a,12,12a,12b-hexadecahydro-cyclobuta[k]cyclo penta[a]phenan thren-3-yl]-ethylideneamino}-ethylamino)-hex-5-yn-1-ol (12)
yielding 64 % of product, m.p. 82-84 oC; IR (Vmax, cm-1): 3400, 3332 and 1058; 1H NMR (300 MHz, CDCl3) δH: 0.06 (s, 6H), 0.88 (s, 3H), 0.90 (s, 9H), 0.98 (s, 3H), 1.16-1.42 (m, 7H), 1.50 (m, 2H), 1.51 (m, 1H), 1.52 (m, 2H), 1.53 (m, 1H), 1.58 (m, 2H), 1.59 (m, 1H), 1.64 (m, 2H), 1.59 (m, 1H), 1.64 (m, 2H), 1.66 (m, 1H), 1.68 (s, 3H), 1.70-1.96 (m, 5H), 2.00 (m, 2H), 2.14-2.28 (m, 4H), 2.30 (m, 2H), 2.40 (m, 1H), 3.18 (m, 2H), 3.50 (m, 1H), 3.54 (m, 2H), 3.58 (broad, 3H), 3.66 (m, 2H), 3.67 (m, 2H), 5.40 (d, 1H, J = 1.32 Hz) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.56 (C-22, C-42), 13.20 (C-24), 16.20 (C-32), 16.90 (C-23), 18.44 (C-43), 21.68 (C-10), 22.62 (C-45), 24.90 (C-38), 25.82 (C-33), 26.10 (C-44, C-46, C-47), 26.40 (C-16), 26.60 (C-15), 28.50 (C-2), 30.10 (C-34), 32.54 (C-39), 33.30 (C-14), 33.80 (C-6), 35.00 (C-3), 35.14 (C-11), 35.57 (C-37), 38.18 (C-9), 38.64 (C-4), 42.82 (C-8), 43.15 (C-13), 44.80 (C-5), 51.12 (C-12), 51.24 (C-27), 53.60 (C-7), 54.16 (C-28), 56.70 (C-17), 62.08 (C-35), 62.50 (C-40), 71.52 (C-1), 78.44 (C-31), 87.32 (C-30), 133.84 (C-19), 153.88 (C-18), 162.70 (C-25) ppm. EI-MS m/z 666.51 Anal. Calcd. for C41H70N2O3Si: C, 73.82; H, 10.58; N, 4.20; O, 7.20; Si, 4.21. Found: C, 73.74; H, 10.44.
Preparation of Cyclobuta-Ynal-Steroid Derivatives
A solution of 10 or 11 or 12 (0.50 mmol), in 5 ml of dimethylsulfoxyde was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, 5 ml of water was added and stirring for 72 h to room temperature. The residue was purified by crystallization from hexane:methanol:water (1:4:2).
7-{2-[8-(tert-Butyl-dimethyl-silanyloxy)-5a,7a-dimethyl-2-(4-oxo-butyl)-4,5,5a,5b,6,7,7a,8,9,10,10a,10b, 11,12-tetradecahydro-2aH-cyclobuta[j]cyclopenta[a]phenanthren-3-ylideneamino]-ethylamino}-hept-6-ynal (13)
yielding 44 % of product, m.p. 50-52 oC; IR (Vmax, cm-1): 3332 and 1058; 1H NMR (300 MHz, CDCl3) δH: 0.08 (s, 6H), 0.69 (s, 3H), 0.84 (s, 9H), 0.92 (m, 1H), 0.98 (s, 3H), 1.02-1.62 (11H), 1.70 (m, 2H), 1.78-1.82 (m, 3H), 1.84 (m, 2H), 1.86 (m, 1H), 1.94 (m, 2H), 2.20-2.22 (m, 2H), 2.24 (m, 2H), 2.26-2.30 (m, 4H), 2.40 (m, 1H), 2.56 (m, 2H), 3.20 (t, 2H, J = 13.34 Hz), 3.52 (m, 1H), 3.56 (t, 2H, J = 8.79 Hz), 5.20 (broad, 1H), 5.22 (m, 1H), 5.40 (d, 1H, J = 1.32 Hz), 9.70 (CHO), 9.82 (CO2H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.60 (C-22, C-42), 11.40 (C-23), 15.60 (C-24), 16.18 (C-31), 17.80 (C-43), 21.00 (C-38), 21.30 (C-13), 22.14 (C-33), 23.54 (C-11), 25.14 (C-9), 25.70 (C-44, C-45, C-46), 26.64 (C-14), 26.94 (C-32), 27.22 (C-15), 31.02 (C-10), 32.06 (C-8), 35.50 (C-37), 36.56 (C-4), 36.70 (C-12), 42.04 (C-7), 43.30 (C-2), 43.52 (C-39), 44.60 (C-34), 45.16 (C-6), 47.40 (C-5), 49.08 (C-17), 51.72 (C-26), 51.98 (C-3), 54.14 (C-27), 79.64 (C-30), 81.66 (C-1), 87.30 (C-29), 132.38 (C-19), 147.80 (C-18), 169.34 (C-16), 202.18 (C-40), 202.44 (C-35) ppm. EI-MS m/z: 648.46 Anal. Calcd. for C40H64N2O3Si: C, 74.02; H, 9.94; N, 4.32; O, 7.40, Si, 4.33. Found: C, 74.00; H, 9.88.
7-[2-(5a,7a-Dimethyl-2-(4-oxo-butyl)-8-{1-[2-(7-oxo-hept-1-ynylamino)-ethylimino]ethyl}-4,5,5a,5b,6,7, 7a,8,9,10,10a,10b,11,12-tetradecahydro-2aH-cyclobuta[j]cyclopen ta[a] phenanthren-3-ylideneamino]-ethylamino]-hept-6-ynal (14)
yielding 64 % of product, m.p. 48-50 oC; IR (Vmax, cm-1): 3330 and 1742; 1H NMR (300 MHz, CDCl3) δH: 0.88 (s, 3H), 0.98 (s, 3H), 1.20-1.56 (m, 10H), 1.68 (s, 3H), 1.70 (m, 4H), 1.78-1.84 (m, 7H), 1.86 (m, 4H), 1.94 (m, 1H), 1.96 (m, 2H), 2.12-2.22 (m, 3H), 2.24 (m, 4H), 2.26 (m, 2H), 2.28 (m, 1H), 2.30 (m, 2H), 2.38-2.40 (m, 2H), 2.56 (m, 4H), 3.16 (m, 4H), 3.56 (m, 4H), 5.20 (broad, 5H), 5.22 (m, 1H), 5.40 (d, 1H, J = 1.82 Hz), 9.70 (CHO), 9.82 (CO2H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 13.20 (C-20), 15.60 (C-21), 16.12 (C-29, C-41), 21.00 (C-48), 21.92 (C-10), 22.10 (C-31, C-43), 22.60 (C-52), 25.18 (C-8), 26.40 (C-12), 26.62 (C-13), 26.64 (C-14), 26.90 (C-30, C-42), 27.28 (C-15), 32.04 (C-7), 35.54 (C-47), 36.52 (C-3), 38.18 (C-9), 42.06 (C-6), 42.82 (C-1), 43.50 (C-49), 44.62 (C-32, C-44), 45.17 (C-5), 48.80 (C-4), 49.02 (C-17), 51.22 (C-24), 51.76 (C-36), 52.44 (C-2), 54.10 (C-25, C-37), 56.70 (C-11), 79.60 (C-28, C-40), 87.28 (C-27, C-39), 132.40 (C-19), 147.80 (C-18), 162.68 (C-22), 169.28 (C-16), 202.20 (C-50), 202.42 (C-33, C-45), ppm. EI-MS m/z 710.51 Anal. Calcd. for C45H66N4O3: C, 76.01; H, 9.36; N, 7.88; O, 6.75. Found: C, 76.00; H, 9.28.
6-(2-{1-[8-(tert-Butyl-dimethyl-silanyloxy)-3a,5b-dimethyl-10-(5-oxo-pentyl) -1,2,3,3a, 4, 5,5a,5b,6,7,8,9, 11a,12,12a,12b-hexadecahydro-cyclobuta[k]cyclopenta[a] phenan- thren-3-yl]ethylideneamino}-ethyl amino)hex-5-ynal (15)
yielding 64 % of product, m.p. 148-150oC; IR (Vmax, cm-1): 3332, 1749 and 1182; 1H NMR (300 MHz, CDCl3) δH: 0.06 (s, 6H), 0.88 (s, 3H), 0.90 (s, 9H), 0.98 (s, 3H), 1.14-1.40 (m, 7H), 1.46 (m, 2H), 1.50-1.52 (m, 2H), 1.56 (m, 2H), 1.58-1.66 (m, 2H), 1.68 (s, 3H), 1.70-1.82 (m, 4H), 1.84 (m, 2H), 1.94 (m, 1H), 1.96 (m, 2H), 2.12-2.28 (m, 4H), 2.34 (m, 2H), 2.40 (m, 1H), 2.44-2.45 (m, 4H), 3.18 (m, 2H), 3.50 (m, 1H), 3.56 (m, 2H), 5.20 (broad, 1H), 5.40 (d, 1H, J = 1.32 Hz), 9.66 (d, 1H, J = 1.90 Hz), 9.72 (d, 1H, J = 1.90 Hz)ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.60 (C-22, C-43), 13.20 (C-24), 15.20 (C-32), 16.90 (C-23), 18.44 (C-44), 21.68 (C-10), 21.72 (C-33), 22.62 (C-46), 23.00 (C-39), 25.30 (C-38), 26.06 (C-45, C-47, C-48), 26.40 (C-16), 26.60 (C-15), 28.52 (C-2), 33.30 (C-14), 33.80 (C-6), 35.00 (C-3), 35.17 (C-11), 35.52 (C-37), 38.18 (C-9), 38.66 (C-4), 42.80 (C-8), 43.12 (C-34), 43.17 (C-13), 43.69 (C-40), 44.80 (C-5), 51.14 (C-12), 51.26 (C-27), 53.60 (C-7), 54.16 (C-28), 56.70 (C-17), 71.56 (C-1), 75.90 (C-31), 87.32 (C-30), 133.94 (C-19), 154.08 (C-18), 162.70 (C-25), 198.62 (C-35), 202.48 (C-41) ppm. EI-MS m/z 676.49 Anal. Calcd. for C42H68N2O3Si: C, 74.50; H, 10.12; N, 4.14; O, 7.09; Si, 4.15. Found: C, 74.42; H, 10.02.
Amination of Cyclobuta-Ynal-Steroid Derivatives
A solution of 13 or 14 or 15 (0.50 mmol), ethylenediamine (60 µl, 0.90 mmol) and boric acid (50 mg, 0.80 mmol), in 5 ml of methanol was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water (3:1).
2-[4-(2-Amino-ethylimino)-butyl]-3-{2-[7-(2-amino-ethylimino)-hept-1-ynylamino]-ethylimino}-8-(tert-butyl-dimethyl-silanyloxy)-5a,7a-dimethyl-2a,3,4,5,5a,5b,6,7,7a,8,9,10,10a,10b,11,12-hexadecahydro-cyclobuta[j]cyclopenta[a]phenanthrene (16).
yielding 63 % of product, m.p. 102-104oC; IR (Vmax, cm-1): 3378, 3330 and 1058; 1H NMR (300 MHz, CDCl3) δH: 0.08 (s, 6H), 0.69 (s, 3H), 0.84 (s, 9H), 0.92 (m, 1H), 0.98 (s, 3H), 1.04-1.56 (9H), 1.58 (m, 2H), 1.60-1.62 (m, 2H), 1.64 (m, 2H), 1.74 (m, 2H), 1.78-1.88 (m, 4H), 2.04 (m, 2H), 2.20 (m, 4H), 2.21-2.24 (m, 2H), 2.28 (m, 2H), 2.36 (m, 2H), 2.40 (m, 1H), 3.10 (m, 4H), 3.20 (t, 2H, J = 13.34 Hz), 3.52 (m, 4H), 3.53 (m, 1H). 3.56 (t, 2H, J = 8.79 Hz), 4.52 (broad, 5H), 5.22 (m, 1H), 5.40 (d, 1H, J = 1.32 Hz), 7.70 (m, 1H), 8.20 (m, 1H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.60 (C-24, C-48), 11.40 (C-21), 15.60 (C-20), 16.38 (C-31), 17.98 (C-49), 21.30 (C-18), 23.54 (C-8), 24.92 (C-34), 25.14 (C-11), 25.22 (C-41), 25.50 (C-50, C-51, C-52), 26.64 (C-12), 26.94 (C-33), 27.22 (C-13), 27.40 (C-32), 31.02 (C-7), 31.10 (C-42), 32.06 (C-10), 36.10 (C-40), 36.56 (C-3), 36.70 (C-19), 40.54 (C-38, C-46), 42.02 (C-9), 43.30 (C-5), 45.16 (C-1), 47.40 (C-2), 49.08 (C-15), 51.62 (C-37), 51.70 (C-26), 51.98 (C-4), 54.14 (C-27), 58.00 (C-45), 79.64 (C-30), 81.66 (C-6), 87.30 (C-29), 132.38 (C-17), 152.24 (C-43), 156.18 (C-35), 156.22 (C-16), 169.34 (C-14) ppm. EI-MS m/z: 732.58 Anal. Calcd. for C44H76N6OSi: C, 72.08; H, 10.45; N, 11.46; O, 2.18, Si, 3.83. Found: C, 72.00; H, 10.34.
(Z)-7-((2-Aminoethyl)imino)-N-(2-(((Z)-1-((5aR,7aS,(S,E)-2-(E)-4-((2-aminoethyl)-imino)butyl)-3-[2-(((Z)-7-((2-aminoethyl)imino)hept-1-yn-1-yl)amino)ethyl)imino)-5a,7a-dimethyl-2a,3,4,5,5a,5b,6,7,7a,8, 9,10,10a,10b,11,12-hexadecahydro-cyclobuta[j]cyclopenta[a]phenanthren-8-yl)ethylidene)amino) ethyl)hept-1-yn-1-amine (19).
yielding 44 % of product, m.p. 84-86oC; IR (Vmax, cm-1): 3378 and 3330; 1H NMR (300 MHz, CDCl3) δH: 0.88 (s, 3H), 0.98 (s, 3H), 1.20-1.56 (m, 10H), 1.58 (m, 4H), 1.66 (m, 4H), 1.68 (s, 3H), 1.74 (m, 2H), 1.78-1.94 (m, 4H), 2.06 (m, 2H), 2.12 (m, 1H), 2.22 (m, 4H), 2.21-2.28 (m, 3H), 2.30 (m, 2H), 2.36 (m, 4H), 2.39-2.40 (m, 2H), 3.10 (m, 6H), 3.16 (m, 4H), 3.52 (m, 4H), 3.56 (m, 4H), 4.60 (broad, 8H), 5.22 (m, 1H), 5.40 (d, 1H, J = 1.82 Hz), 7.70 (m, 2H), 8.20 (m, 1H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 13.20 (C-21), 15.60 (C-20), 16.42 (C-28, C-52), 21.92 (C-7), 22.60 (C-61), 24.90 (C-31, C-55), 25.18 (C-10), 25.22 (C-17, C-38), 26.40 (C-18), 26.62 (C-11), 27.00 (C-30, C-54), 27.28 (C-12), 27.44 (C-29, C-53), 31.10 (C-39), 32.04 (C-9), 36.14 (C-37), 36.52 (C-3), 38.28 (C-6), 40.56 (C-35, C-43, C-59), 42.02 (C-8), 42.82 (C-5), 45.20 (C-1), 48.80 (C-2), 49.02 (C-14), 51.22 (C-47), 51.60 (C-34, C-58), 51.76 (C-23), 54.10 (C-24, C-48), 56.20 (C-4), 56.70 (C-19), 58.00 (C-42), 79.60 (C-27, C-51), 87.28 (C-26, C-50), 132.40 (C-16), 152.26 (C-40), 156.20 (C-32, C-56), 156.28 (C-15), 162.68 (C-45), 169.28 (C-13) ppm. EI-MS m/z 836.68 Anal. Calcd. for C51H84N10: C, 73.16; H, 10.11; N, 16.73. Found: C, 73.07; H, 10.04.
(E)-6-((2-Aminoethyl)imino)-N-(2-(((Z)-1-((3R,3aS,5bR,8S)-10-((E)-5-((2-aminoethyl)- imino)pentyl)-8-(((tert-butyldimethylsilyl)oxy)-3a,5b-dimethyl-1,2,3,3a4,5,5a,5b,6,7,8,9,11a,12,12a,12b-hexadecahydro cyclobuta[k]cyclopenta[a]phenanthren-3-yl)ethylidene)amino)ethyl)hex-1-yn-1-amine (21).
yielding 45 % of product, m.p. 142-144oC; IR (Vmax, cm-1): 3378, 3330 and 1058; 1H NMR (300 MHz, CDCl3) δH: 0.06 (s, 6H), 0.88 (s, 3H), 0.90 (s, 9H), 0.98 (s, 3H), 1.14-1.38 (m, 6H), 1.40 (m, 2H), 1.41-1.66 (m, 5H), 1.68 (s, 3H), 1.70 (m, 1H), 1.76 (m, 2H), 1.78-1.84 (m, 3H), 1.90 (m, 2H), 1.94 (m, 1H), 2.06-2.10 (m, 4H), 2.12 (m, 2H), 2.14-2.40 (m, 5H), 2.42 (m, 2H), 3.10 (m, 4H), 3.18 (m, 2H), 3.52 (m, 4H), 3.54 (m, 1H), 3.56 (m, 2H), 4.52 (broad, 5H), 5.40 (d, 1H, J = 1.32 Hz), 8.10 ((m, 2H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.60 (C-24, C-49), 13.20 (C-21), 16.80 (C-32), 16.90 (C-20), 18.44 (C-50), 21.68 (C-7), 22.62 (C-52), 25.30 (C-15), 26.06 (C-51, C-53, C-54), 26.40 (C-16), 26.80 (C-41), 27.46 (C-33), 27.82 (C-42), 28.50 (C-9), 30.30 (C-34), 30.40 (C-43), 33.30 (C-14), 33.80 (C-3), 35.00 (C-8), 35.17 (C-11), 35.82 (C-40), 38.28 (C-6), 38.66 (C-1), 40.50 (C-38, C-47), 42.80 (C-5), 43.17 (C-13), 44.80 (C-2), 51.14 (C-12), 51.26 (C-27), 54.16 (C-28), 56.70 (C-17), 57.34 (C-4), 58.00 (C-37, C-46), 71.56 (C-10), 86.46 (C-31), 87.32 (C-30), 133.94 (C-19), 154.08 (C-18), 154.50 (C-35, C-44), 162.70 (C-25) ppm. EI-MS m/z 760.61 Anal. Calcd. for C46H80N6OSi: C, 72.58; H, 10.59; N, 11.04; O, 2.10; Si, 3.69. Found: C, 72.42; H, 10.42.
Removal of the tert-butyldimethylsilylane fragment of 16 or 21
A solution of 16 or 21 (0.50 mmol) in 5 ml of hydrofluoric acid was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water (3:1).
2-[4-(2-Amino-ethylimino)butyl]-3-{2-[7-(2-amino-ethylimino)-hept-1-ynylamino]-ethylimino}-5a,7a-dimethyl-2a,3,4,5,5a,5b,6,7,7a,8,9,10,10a,10b,11,12-hexadecahydro-cyclobuta[j]cyclopenta[a] phenanthren-8-ol (17)
yielding 55 % of product, m.p. 100-102oC; IR (Vmax, cm-1): 3400, 3380 and 3330; 1H NMR (300 MHz, CDCl3) δH: 0.84 (s, 3H), 0.94 (m, 1H), 0.98 (s, 3H), 1.10-1.56 (10H), 1.58 (m, 2H), 1.62 (m, 1H), 1.66 (m, 2H), 1.74 (m, 2H), 1.78-1.98 (m, 4H), 2.04 (m, 2H), 2.20 (m, 2H), 2.21-2.24 (m, 2H), 2.30 (m, 2H), 2.36 (m, 2H), 2.40 (m, 1H), 3.10 (m, 4H), 3.20 (t, 2H, J = 13.34 Hz), 3.52 (m, 4H), 3.56 (t, 2H, J = 8.79 Hz), 3.64 (m, 1H), 4.80 (broad, 6H), 5.22 (m, 1H), 5.40 (d, 1H, J = 1.32 Hz), 7.70 (m, 1H), 8.20 (m, 1H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 11.80 (C-21), 15.60 (C-20), 16.38 (C-29), 21.30 (C-18), 23.74 (C-8), 24.92 (C-32), 25.14 (C-11), 25.22 (C-39), 26.64 (C-12), 26.94 (C-31), 27.22 (C-13), 27.40 (C-30), 30.72 (C-7), 31.10 (C-40), 32.06 (C-10), 36.10 (C-38), 36.56 (C-3), 37.30 (C-19), 40.54 (C-36, C-44), 42.02 (C-9), 44.00 (C-5), 45.16 (C-1), 47.40 (C-2), 49.08 (C-15), 51.12 (C-4), 51.62 (C-35), 51.78 (C-24), 54.14 (C-25), 58.00 (C-43), 79.64 (C-28), 81.80 (C-6), 87.30 (C-27), 132.38 (C-17), 152.24 (C-41), 156.18 (C-33), 156.22 (C-16), 169.34 (C-14) ppm. EI-MS m/z: 618.49 Anal. Calcd. for C38H62N6O: C, 73.74; H, 10.10; N, 13.58; O, 2.58. Found: C, 73.66; H, 10.04.
3-(1-{2-[6-(2-Aminoethylimino)-hex-1-ynylamino]ethylimino}-ethyl)-10-[5-(2-amino-ethylimino)-penthyl]-3a,5b-dimethyl-1,2,3,3a4,5,5a,5b,6,7,8,9,11a,12,12a,12b-hexadecahydrocyclobuta[k]cyclo penta[a]phenanthren-8-ol (22)
yielding 38 % of product, m.p. 122-124oC; IR (Vmax, cm-1): 3402 and 33323; 1H NMR (300 MHz, CDCl3) δH: 0.88 (s, 3H), 0.98 (s, 3H), 1.14-1.38 (m, 6H), 1.40 (m, 2H), 1.42-1.66 (m, 5H), 1.68 (s, 3H), 1.70-1.74 (m, 2H), 1.76 (m, 2H), 1.78-1.84 (m, 2H), 1.90 (m, 2H), 1.94 (m, 1H), 2.06-2.08 (m, 4H), 2.12 (m, 2H), 2.14-2.40 (m, 5H), 2.42 (m, 2H), 3.10 (m, 4H), 3.18 (m, 2H), 3.52 (m, 4H), 3.56 (m, 2H), 3.80 (m, 1H), 4.24 (broad, 6H), 6.00 (d, 1H, J = 1.00 Hz), 7.70 (m, 1H), 8.10 (m, 1H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 13.20 (C-21), 16.80 (C-30), 16.90 (C-20), 21.68 (C-47), 22.62 (C-47), 24.00 (C-41), 25.30 (C-15), 26.40 (C-16), 26.80 (C-39), 27.46 (C-31), 27.82 (C-40), 30.30 (C-32), 30.90 (C-9), 33.33 (C-14), 33.66 (C-8), 33.80 (C-3), 34.80 (C-11), 35.82 (C-38), 38.28 (C-6), 38.66 (C-1), 40.50 (C-36, C-45), 42.80 (C-5), 43.17 (C-13), 44.80 (C-2), 51.24 (C-25), 51.26 (C-12), 51.62 (C-44), 54.16 (C-26), 56.70 (C-17), 57.34 (C-4), 58.00 (C-35), 69.70 (C-10), 86.46 (C-29), 87.32 (C-28), 133.94 (C-19), 154.08 (C-18), 154.50 (C-33), 156.20 (C-42), 162.70 (C-23) ppm. EI-MS m/z 646.52 Anal. Calcd. for C40H66N6O: C, 74.26; H, 10.28; N, 12.99; O, 2.47. Found: C, 74.13; H, 10.14.
Preparation of 1,4-diazacycloundeca-5,11-dien-steroid derivatives
A solution of 17 or 19 or 22 (0.50 mmol), Copper(II) chloride anhydrous (70 mg, 0.52 mmol), in 5 ml of methanol was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water (3:1).
2-[4-(2-Amino-ethylimino)butyl]-3-[2-(1,4-diaza-cycloundeca-5,11-dien-5-ylamino)-ethylimino]-5a,7a-dimethyl-2a,3,4,5,5a,5b,6,7,7a,8,9,10,10a,10b,11,12-hexadecahydro-cyclobuta[j]cyclopenta[a]phenan thren-8-ol (18)
yielding 55 % of product, m.p. 116-118oC; IR (Vmax, cm-1):3402, 3380 and 3330; 1H NMR (300 MHz, CDCl3) δH: 0.84 (s, 3H), 0.94 (m, 1H), 0.98 (s, 3H), 1.10-1.64 (11H), 1.74 (m, 2H), 1.78-1.82 (m, 2H), 1.84 (m, 2H), 1.86 (m, 1H), 1.88 (m, 2H), 1.98 (m, 1H), 2.04 (m, 2H), 2.06 (m, 2H), 2.21-2.24 (m, 2H), 2.30 (m, 2H), 2.32 (m, 2H), 2.40 (m, 1H), 3.10 (m, 2H), 3.12 (m, 2H), 3.48 (m, 2H), 3.52-3.58 (m, 4H), 3.64 (m, 1H), 3.90 (m, 2H), 4.44 (d, 1H, J = 0.78 Hz), 5.22 (m, 1H), 5.40 (d, 1H, J = 1.32 Hz), 5.50 (broad, 5H), 6.70 (m, 1H), 8.20 (m, 1H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 11.80 (C-36), 15.60 (C-35), 21.30 (C-33), 23.74 (C-26), 25.14 (C-32), 25.22 (C-39), 26.64 (C-28), 27.22 (C-7), 27.25 (C-27), 28.88 (C-8), 30.72 (C-25), 31.10 (C-40), 32.06 (C-31), 32.70 (C-10), 34.12 (C-9), 36.10 (C-38), 36.56 (C-21), 37.30 (C-34), 40.54 (C-44), 42.02 (C-18), 43.60 (C-3), 44.00 (C-23), 45.16 (C-19), 45.87 (C-13), 47.40 (C-20), 49.08 (C-17), 51.12 (C-22), 52.72 (C-14), 58.00 (C-43), 61.27 (C-2), 73.34 (C-6), 81.80 (C-24), 132.38 (C-30), 149.60 (C-5), 152.24 (C-41), 156.28 (C-29), 159.88 (C-11), 169.34 (C-16) ppm. EI-MS m/z: 618.49 Anal. Calcd. for C38H62N6O: C, 73.74; H, 10.10; N, 13.58; O, 2.58. Found: C, 73.62; H, 10.02.
(1Z,5E)-N-(2-(((Z)-1-((5aR,7aS,8S,E)-3-((2-(((1E,5Z)-1,4-diazacycloundeca-5,11-dien-5-yl)amino)ethyl) imino)-2-(E)-4-((2-aminoethyl)imino)butyl)-5a,7a-dimethyl-2a,3,4,5,5a,5b,6,7,7a,8,9,10,10a,10b,11,12-hexadecahydro-cyclobuta[j]cyclopenta[a]phenanthren-8-yl)ethylidene)amino)ethyl)-1,4-diazacycloun deca-5,11-dien-5-amine (20)
yielding 66 % of product, m.p. 62-64oC; IR (Vmax, cm-1): 2402, 3376 and 3330; 1H NMR (300 MHz, CDCl3) δH: 0.88 (s, 3H), 0.98 (s, 3H), 1.20-1.58 (m, 10H), 1.68 (s, 3H), 1.74 (m, 2H), 1.78-1.82 (m, 3H), 1.86 (m, 4H), 1.88 (m, 4H), 1.94 (m, 1H) 2.04 (m, 2H), 2.06 (m, 4H), 2.12-2.28 (m, 4H), 2.29 (m, 2H), 2.30 (m, 4H), 2.39-2.40 (m, 2H), 2.80 (m, 2H), 3.10 (m, 2H), 3.14 (m, 4H), 3.46 (m, 4H), 3.52 (m, 2H), 3.56-3.58 (m, 4H), 3.90 (m, 2H), 3.96-4.42 (m, 2H), 5.22 (m, 1H), 5.40 (d, 1H, J = 1.82 Hz), 5.60 (broad, 6H), 6.70 (m, 2H), 8.20 (m, 1H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 13.20 (C-36), 15.60 (C-35), 21.92 (C-25), 22.60 (C-61), 25.18 (C-31), 25.22 (C-32), 25.24 (C-38), 26.30 (C-56), 26.40 (C-33), 26.62 (C-27), 26.64 (C-27), 27.20 (C-7, C-59), 27.28 (C-26), 28.84 (C-8, C-8, 31.10 (C-39), 32.04 (C-30), 32.70 (C-10), 34.16 (C-9, C-57), 36.14 (C-37), 36.52 (C-21), 38.28 (C-28), 40.56 (C-43), 42.02 (C-18), 42.82 (C-23), 43.60 (C-3, C-52), 45.20 (C-19), 45.86 (C-13, C-48), 48.80 (C-20), 49.02 (C-17), 52.22 (C-47), 52.76 (C-14), 54.90 (C-53), 56.20 (C-22), 56.70 (C-34), 58.00 (C-42), 61.26 (C-2), 73.40 (C-6, C-60), (C-18), 132.40 (C-29), 149.60 (C-5, C-50), 152.26 (C-40), 156.20 (C-28), 159.90 (C-11, C-55), 162.68 (C-45), 169.28 (C-16) ppm. EI-MS m/z 836.68 Anal. Calcd. for C51H84N10: C, 73.16; H, 10.11; N, 16.73. Found: C, 73.08; H, 10.02.
10-[5-(2-Aminoethylimino)-penthyl]-3-{1-[2-(1,4-diaza-cycloundeca-5,11-dien-5-ylamino)-ethylimino]-ethyl}-3a,5b-dimethyl-1,2,3,3a4,5,5a,5b,6,7,8,9,11a,12,12a,12b-hexadecahydrocyclobuta[k]cyclopenta [a]phenanthren-8-ol (23).
yielding 64 % of product, m.p. 150-152 oC; IR (Vmax, cm-1): 3380 and 3332; 1H NMR (300 MHz, CDCl3) δH: 0.88 (s, 3H), 0.98 (s, 3H), 1.14-1.38 (m, 6H), 1.40 (m, 2H), 1.42-1.66 (m, 5H), 1.68 (s, 3H), 1.70-1.84 (m, 4H), 1.86-1.88 (m, 4H), 1.90 (m, 2H), 1.94 (m, 1H), 2.04 (m, 2H), 2.06-2.10 (m, 4H), 2.12-2.28 (m, 4H), 2.30 (m, 2H), 2.40 (m, 1H), 2.78 (m, 2H), 3.10 (m, 2H), 3.12 (m, 2H), 3.48 (m, 2H), 3.52 (m, 2H), 3.56 (m, 2H), 3.80 (m, 1H), 3.96 (m, 1H), 4.80 (broad, 5H), 5.40 (d, 1H, J = 0.70 Hz), 6.70 (m, 1H), 8.10 (m, 1H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 13.20 (C-37), 16.90 (C-38), 21.68 (C-33), 22.62 (C-36), 25.30 (C-31), 26.30 (C-10), 26.40 (C-30), 26.80 (C-41), 27.20 (C-7), 27.82 (C-42), 28.88 (C-8), 30.40 (C-43), 30.90 (C-24), 33.33 (C-29), 33.66 (C-23), 33.80 (C-9), 34.12 (C-9), 34.80 (C-26), 35.82 (C-40), 38.28 (C-32), 38.66 (C-22), 40.50 (C-47), 42.80 (C-47), 42.80 (C-18), 43.17 (C-28), 43.60 (C-3), 44.80 (C-21), 45.82 (C-13), 51.24 (C-27), 52.26 (C-14), 54.88 (C-2), 56.70 (C-17), 57.34 (C-19), 58.00 (C-46), 69.70 (C-25), 73.40 (C-6), 133.94 (C-35), 149.60 (C-5), 154.08 (C-34), 154.50 (C-44), 159.90 (C-11), 162.70 (C-16) ppm. EI-MS m/z 646.52 Anal. Calcd. for C41H68N6O: C, 74.26; H, 10.37; N, 12.71; O, 2.42. Found: C, 74.14; H, 10.24.
Antimicrobial Activity
The evaluation of antimicrobial effect of the different compounds on the bacterial species was made by a previously method described21. The bacterial species were incubated on brain/heart Infusion (Streptococcus pneumoniae) and Staphylococcus 110 (Staphylococcus aureus) agars for 24 h at 37°C. After such time, it was be determined whether growth had taken place or not. In addition, a series of tubes were prepared, the first of which contained 2 ml of culture medium (tripticase soye) at double concentration and the remainder (11tubes), contained the same quantity of medium at single concentrations. From the first tube (double concentration) an aliquot of 2 ml of the studied compound (1 mg/ml) was added and stirred, from this tube an aliquot of 2 ml was taken and added to the following tube (simple concentration) and the process was successively repeated until the last 2 ml of dissolution had been used up. After this process, each tube was inoculated with 0.1 ml of the bacterial suspension, whose concentration corresponded to Mc-Farland scale (9 ´108 cells/ml) and all the tubes were incubated at 37°C for 24 h. Subsequently, a loop was taken from each of them and inoculated into the appropriate cultures for different bacterial organisms, and were incubated for 24 h at 37°C. After such time, the minimum inhibitory concentration (MIC) was evaluated to consider the antimicrobial effect of the different compounds. In order to discard the effect of methanol (solvent) on the bacterial species studied, a series of the same number of tubes was prepared in parallel, to which 2 ml of methanol at 60% was added to the first and corresponding successive dilutions were added in the same way as before. In addition a control series was also performed using distilled water to pH 7.0.
Docking Server
Docking calculations were carried out using Docking Server22. The MMFF94 force field23 was used for energy minimization of ligand molecule using the Docking Server. Gasteiger partial charges were added to the ligand atoms. Non-polar hydrogen atoms were merged, and rotatable bonds were defined. Docking calculations were carried out on the HERD224 and PARP25 protein model. Essential hydrogen atoms, Kollman united atom type charges, and solvation parameters were added with the aid of AutoDock tools [26]. Affinity (grid) maps of 20 × 20 × 20-Å grid points and 0.375-Å spacing were generated using the Autogrid program26. AutoDock parameter set and distance dependent dielectric functions were used in the calculation of the Vander Waals and the electrostatic terms, respectively. Docking simulations were performed using the Lamarckian genetic algorithm (LGA) and the Solis and Wets local search method27. Initial position, orientation, and torsions of the ligand molecules were set randomly. Each docking experiment was derived from two different runs that were set to terminate after a maximum of 250,000 energy evaluations. The population size was set to 150. During the search, a translational step of 0.2Å and quaternion and torsion steps of 5 were applied.
Evaluation of Lipophilicity Degree of Compounds 18, 20 and 23
To estimate the logarithmic octanol-water partition coefficient (log P) of compounds 18, 20 and 23, the logKow method (atom/fragment contribution), introduced by Mannhold and Howard (method A), available as the KOWWIN and KLogP (method B) software’s and the fragmental technique ACDLogP (method C) were used28.
Statistical Analysis
The obtained values are expressed as average ± SE, using each heart (n = 9) as its own control. The data obtained were put under Analysis of Variance (ANOVA) with the Bonferroni correction factor29 using the SPSS 12.0 program. The differences were considered significant when p was equal or smaller than 0.05.
Statistical Analysis
The obtained values are expressed as average ± SE, using each heart (n = 9) as its own control. The data obtained were put under Analysis of Variance (ANOVA) with the Bonferroni correction factor29 using the SPSS 12.0 program. The differences were considered significant when p was equal or smaller than 0.05.
Results and Discussion
There are reports which indicate the preparation of diazahicyclo derivatives as antibacterial agents; nevertheless, expensive reagents and special conditions are required9-17. Therefore, in this study three diazahicyclo-steroid derivatives were synthetized using several strategies to evaluate the biological activity against Staphylococcus aureus and Streptococcus pneumoniae
Preparation of Three Diazecin-Steroid-Hexahydroazocin Derivatives
In this study several straightforward routes are reported for synthesis of three diazahicyclo-steroid derivatives using OTBS-testosterone (1), progesterone (2) and OTBS-pregnenolone (3) as chemical tools (Scheme 1). The first stage was achieved by the synthesis of three cyclobutane-steroid derivatives (4 or 5 or 6, Scheme 2); it is important to mention that there are several reports to preparation of cyclobutene rings using some reagents such as Co(PPh3)2I2/PPh3/Zn30, rodhium31, nikel32, ruthenium33 and others. In this study, the compounds 1, 2 and 3 were reacted with 5-hexyn-1-ol using Cooper II chloride as catalyst to form 4 or 5 or 6. The 1H NMR spectrum of 4 shows signals at 0.08 and 0.86 ppm for ter-butyldimethylsylane fragment; at 0.68 and 1.08 for methyl groups bound to steroid nucleus; at 0.94-1.04, 1.20-1.48, 1.62-2.10 and 2.22-3.54 ppm for steroid moiety; at 1.52-1.60, 2.20 and 3.66 ppm for methylene groups involved in the arm bound to A-ring; at 5.70 ppm for cyclobutane. The 13C NMR spectra showed chemical shifts at -4.60, 18.02 and 25.50 ppm for ter-butyldimethylsylane fragment; at 11.32-14.80 ppm for methyl groups bound to steroid nucleus; at 21.34-23.52, 25.24, 31.06-32.44, 36.54-36.70, 38.50-60.16 and 81.62 ppm for steroid moiety; at 24.30, 32.54, 37.02 and 62.5 ppm for methylene groups involved in the arm bound to A-ring; at 134.52-147.68 ppm for cyclobutane ring; at 210.62 ppm for ketone group. Finally, the presence of 4 was further confirmed from mass spectrum which showed a molecular ion at m/z: 500.36.
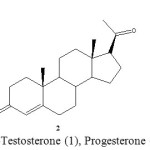 |
Scheme 1: Chemical structure of OTBS-Testosterone (1), Progesterone (2) and OTBS-pregnenolone (3). Click here to View scheme |
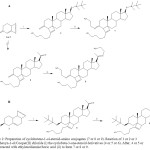 |
Scheme 2: Preparation of cyclobutene-1-ol-steroid-amino conjugates (7 or 8 or 9). Reaction of 1 or 2 or 3 with 5-hexyn-1-ol/Cooper(II) chloride (i) the cyclobuta-3-one-steroid derivatives (4 or 5 or 6). After, 4 or 5 or 6 were reacted with ethylenediamine/boric acid (ii) to form 7 or 8 or 9. |
On the other hand, the 1H NMR spectrum of 5 showed characteristic signals at 2.12 for methyl group bound to ketone; 3.60 ppm for hydroxyl group; at 5.70 ppm for cyclobutane ring. The 13C NMR spectra showed chemical shifts at 30.74 ppm for methyl group bound to ketone; at 134.52-147.68 ppm for cyclobutane ring; at 208.28-210.62 ppm for ketone groups. In addition, the presence of 5 was further confirmed from mass spectrum which showed a molecular ion at m/z: 412.29.
Finally, the 1H NMR spectrum of 6 shows signals at 1.501.52, 2.02 and 3.66 ppm for methylene groups involved in the arm bound to A-ring; at 3.60 ppm for hydroxyl group at 5.40 for cyclobutane ring. The 13C NMR spectra showed chemical shifts at 28.34 ppm for methyl group bound to ketone; at 24.90 and 32.54, 35.62, 62.55 and 71.56 ppm for methylene groups involved in the arm bound to A-ring; at 133.92-153.90 ppm for cyclobutane ring; at 208.58 ppm for ketone group. Finally, the presence of 6 was further confirmed from mass spectrum which showed a molecular ion at m/z: 528.39.
Preparation of Cyclobutene-Ol-Steroid-Amino Conjugates
The following stage was achieved by preparation of imino groups involved in the compounds 7 or 8 or 9 (Scheme 2). It is important to mention, that there are several procedures for the synthesis of imino groups which are described in the literature34, 35. In this study the compounds 7 or 8 or 9 were synthesized (Figure 3) by the reaction of 3 or 5 or 6 with ethylenediamine using boric acid as catalyst, because it is not an expensive reagent and no special conditions are required for use36. The 1H NMR spectrum of 7 showed signals at 1.50, 1.62, 2.15 and 3.66 ppm for methylene groups of arm bound to cylobutene ring; at 3.10-3.50 ppm for methylene groups involved in the arm bound to A-ring of steroid; at 4.10 ppm for both hydroxyl and amino groups; at 5.40 for cyclobutane ring. The 13C NMR spectra showed chemical shifts at 24.38, 32.54-36.50 and 62.55 ppm for methylene groups of arm bound to cyclobutane ring; at 41.00 and 53.60 ppm for methylene groups involved in the arm bound to A-ring of steroid; at 132.34-150.38 for cyclobutane ring; at 169.34 ppm for imino group. In addition, the presence of 7 was further confirmed from mass spectrum which showed a molecular ion at m/z: 542.42.
On the other hand, the 1H NMR spectrum of 8 showed signals at 1.50, 1.62, 2.18 and 3.66 ppm for methylene groups of arm bound to cyclobutane ring; at 3.09 and 3.52 ppm for methylene groups bound to both amino and imino groups; at 3.10-3.50 ppm for methylene groups involved in the arm bound to A-ring of steroid; at 4.20 ppm for both hydroxyl and amino groups; at 5.40 ppm for cyclobutane ring. The 13C NMR spectra showed chemical shifts at 24.40, 32.54-36.52 and 62.55 ppm for methylene groups of arm bound to cyclobutane ring; at 41.00 and 53.60 ppm for methylene groups involved in the arm bound to A-ring of steroid; at 41.02 and 53.10 ppm for methylene groups bound to both amino and imino groups; at 132.32-150.38 ppm for cyclobutane ring; at 156.78-169.28 ppm for imino group. In addition, the presence of 8 was further confirmed from mass spectrum which showed a molecular ion at m/z: 496.41.
Finally, other results showed several signals involved in the 1H NMR spectrum for 9 at; 0.07 and 0.90 ppm for ter-butyldimethylsylane fragment; at 1.50, 1.52 and 2.00 ppm for methylene groups of arm bound to cyclobutane ring; at 3.08-3.52 and 3.66 ppm for methylene groups involved in the arm bound to A-ring of steroid; at 3.08-3.52 and 3.66 ppm for methylene groups bound to both imino and amino groups; at 4.10 ppm for both hydroxyl and amino groups; at 5.40 ppm for cyclobutane ring. The 13C NMR spectra showed chemical shifts at 29.90, 32.54, 35.57 and 62.55 ppm for methylene groups of arm bound to cyclobutane ring; at 41.00 and 53.10 ppm for methylene groups bound to both imino and amino groups; at 133.94-153.88 ppm for cyclobutene ring; at 156.70 ppm for imino group. In addition, the presence of 9 was further confirmed from mass spectrum which showed a molecular ion at m/z: 570.45.
Alkynylation of Amino Groups
There are some studies which shown the reaction of chloro-hexyne derivatives with secondary amines; for example, the preparation of β-Alkynyl-β-amino Esters via the Mannich reaction with silylketene acetals and alkynyl imines using silver as catalyst37.. Other data indicate the preparation of an indole-alkyne derivative by the reaction of 5-chloro-1-pentyne or 6-chloro-1-hexyne with indole-3-acetamide in basic medium38. In this investigation the compounds 7, 8 or 9 were reacted with 5-hexyn-2-ol in presence of CopperII chloride to form 10 or 11 or 12 (Scheme 3). The mechanism involves the compounds 7 or 8 via SN2 mechanism (Fig.4 and 5). The 1H NMR spectrum of 10 showed signals at 1.58, 1.64, 2.30 and 3.66 ppm for methylene groups bound to both alkyne and hydroxyl groups; at 1.68, 2.26 and 3.52 ppm for methylene groups of arm bound to cyclobutene ring; at 3.20 and 3.56 ppm for methylene groups bound to both amino and imino groups; at 3.80 ppm for both hydroxyl and amino groups; at 5.40 for cyclobutene ring. The 13C NMR spectra showed chemical shifts at 23.14, 33.40 and 61.86 for methylene groups of arm bound to cyclobutene ring; at 51.72 and 54.14 ppm for methylene groups bound to both amino and imino groups; at 78.44 and 87.30 ppm for alkyne group; at 133.52-150.58 ppm for cyclobutene ring; at 169.34 ppm for imino group. Finally, the presence of 10 was further confirmed from mass spectrum which showed a molecular ion at m/z: 624.46.
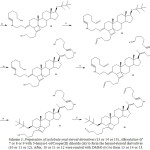 |
Scheme 3: Preparation of cyclobuta-ynal-steroid derivatives (13 or 14 or 15). Alkynylation 0f 7 or 8 or 9 with 5-hexyn-1-ol/Cooper(II) chloride (iii) to form the heynol-steroid derivatives (10 or 11 or 12). Click here to View scheme |
After, 10 or 11 or 12 were reacted with DMSO (iv) to form 13 or 14 or 15.
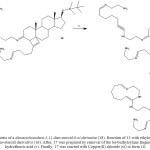 |
Scheme 4: Synthesis of a diazacycloundeca-5,11-dien-steroid-8-ol derivative (18). Reaction of 13 with ethylenediamine (v) to form an amino-steroid derivative (16). Click here to View scheme |
After, 17 was prepared by removal of the ter-buthylsylane fragment of 16 with hydrofluoric acid (v). Finally, 17 was reacted with Copper(II) chloride (vi) to form 18.
On the other hand, the 1H NMR spectrum of 11 showed signals at 1.58-1.66, 2.30 and 3.64 ppm for methylene groups bound to both hydroxyl and alkyne groups; at 1.69, 2.26 and 3.52 ppm for methylene groups involved in the arm bound to cyclobutene ring; at 3.16 and 3.56 ppm for methylene groups bound to both amino and imino groups; at 3.70 ppm for both hydroxyl and amino groups; at 5.40 for cyclobutene ring. The 13C NMR spectra showed chemical shifts at 23.20, 33.42 and 61.80 ppm for methylene groups involved in the arm bound to cyclobutene ring; at 51.22-51.76 and 54.10 ppm for methylene groups bound to both amino and imino groups; at 78.48-87.28 ppm for alkyne group at 133.52-150.58 ppm for cyclobutene ring; at 162.68- 169.28 ppm for imino groups. In addition, the presence of 11 was further confirmed from mass spectrum which showed a molecular ion at m/z: 674.51.
Finally, The 1H NMR spectrum of 12 showed signals at 0.06 and 0.90 ppm for ter-butyldimethylsylane fragment; at 1.52, 2.00 and 3.66 ppm for methylene groups involved in the arm bound to cyclobutene ring; at 1.58, 1.64, 2.30 and 3.67 ppm for methylene groups bound to both hydroxyl and alkyne groups; at 3.18 and 3.54 ppm for methylene groups bound to both amino and imino groups; at 3.58 ppm for both hydroxyl and amino groups; at 5.40 ppm for cyclobutene ring. The 13C NMR spectra showed chemical shifts at 16.20, 25.82, 30.10 and 62.08 ppm for methylene groups bound to both hydroxyl and alkyne groups; at 24.90, 32.54, 35.52 and 62.50 ppm for methylene groups involved in the arm bound to cyclobutene ring; at 51.24 and 56.16 ppm for methylene groups bound to both amino and imino groups; at 78.44-87.32 ppm for alkyne group; at 133.84-153.88 ppm for cyclobutene ring; at 162.70 ppm for imino group. Finally, the presence of 12 was further confirmed from mass spectrum which showed a molecular ion at m/z: 666.51.
Preparation of Aldehyde-Steroid Derivative
The sixth stage was achieved by the synthesis of a aldehyde-steroid derivatives (13 or 14 or 15, Scheme 3); it is noteworthy that there are several reports on the oxidation of primary alcohols to form the corresponding aldehydes. In addition, some reports indicate the preparation of aldehyde derivatives using several reagents such as morpholinium bisulfate39, calcium hydride40, 2-(hydroxyalky1)dithianes41, KN(TMS)242, chromium(VI)43, ruthenium44 and others. However, some these reagents may induce risks of toxicity by generation of several substances involved on the reaction mixtures. Therefore, in this study a method previously reported37 for oxidation of hydroxyl groups was used for synthesis of 13 or 14 or 15 by the reaction of 10 or 11 or 12 with dimethyl sulfoxide. The 1H NMR spectrum of 13 shows signals at 1.70, 1.84, 2.24 and 2.56 ppm for methylene groups bound to both aldehyde and alkyne groups; at 1.94 and 2.26-2.30 ppm for methylene groups involved in the arm bound to both aldehyde and cyclobutene ring; at 3.20 and 3.56 for methylene groups bound to both amino and imino groups; at 5.20 ppm amino group; at 5.40 ppm for cyclobutene ring; at 9.70-9.82 ppm for aldehyde groups. The 13C NMR spectra showed chemical shifts at 16.18, 22.14, 26.94 and 44.52 ppm for methylene groups involved in the arm bound to both aldehyde and cyclobutene ring; at 51.72 and 54.14 for methylene groups bound to both amino and imino groups; at 79.64 and 87.30 ppm for alkyne group; at 132.38-147.80 for cyclobutene ring; at 169.34 ppm for imino group; at 202.18-202.44 for aldehyde groups. In addition, the presence of 13 was further confirmed from mass spectrum which showed a molecular ion at m/z: 666.51.
Other signals of 1H NMR spectrum for 14 were found at 1.68 ppm for methyl group bound to imino group; at 1.70, 1.86, 2.24 and 2.56 ppm for methylene groups bound to both aldehyde and alkyne groups; at 1.96, 2.26 and 2.30 ppm for methylene groups involved in the arm bound to both aldehyde and cyclobutene ring; at 3.16 and 3.56 ppm for methylene groups bound to both amino and imino groups; at 5.20 ppm for amino group; at 5.40 ppm for cyclobutene group; at 9.70-9.82 ppm for aldehyde groups. The 13C NMR spectra showed chemical shifts at 16.12, 22.10 and 26.90 ppm for methylene groups bound to both alkyne and aldehyde groups; at 21.00, 35.54, 43.50 and 44.62 ppm for methylene groups involved in the arm bound to both aldehyde and cyclobutene ring; at 51.22-51.76 and 54.10 ppm for methylene groups bound to amino and imino groups; at 79.60-87.28 ppm for alkyne group; at 132.40-147.80 ppm for cyclobutene ring; at 162.68-169.28 ppm for imino groups; at 202.20-202.42 ppm for aldehyde groups. Finally, the presence of 14 was further confirmed from mass spectrum which showed a molecular ion at m/z: 710.51.
Other results indicated that 1H NMR spectrum of 15 shows signals at 1.84 and 2.44-2.45 ppm for methylene groups bound to both alkyne and aldehyde groups; at 1.46 and 1.56, 1.96 and 2.34 ppm for methylene groups involved in the arm bound to both aldehyde and cyclobutene ring; at 3.18 and 3.56 for methylene groups bound to both amino and imino groups; at 5.20 ppm for amino group; at 5.40 for cyclobutene ring; at 9.66-9.72 ppm for aldehyde groups. The 13C NMR spectra showed chemical shifts at 15.20, 21.72 and 43.12 ppm for methylene groups bound to both alkyne and aldehyde groups; at 23.00-25.30, 35.52 and 43.69 ppm for methylene groups involved in the arm bound to both aldehyde and cyclobutene ring; at 51.26 and 54.16 ppm for methylene groups bound to both amino and imino groups; at 133.90-154.08 ppm for cyclobutene ring; at 162.70 ppm for imino group; at 198.62-202.48 ppm for aldehyde groups. Finally, the presence of 15 was further confirmed from mass spectrum which showed a molecular ion at m/z: 666.51.
Preparation of Imino-Steroid Derivatives (Scheme 4, 5 and 6)
The compounds 13 or 14 or 15 were reacted with ethylenediamine to form the compounds 16 or 19 or 21 using boric acid as catalyst. The 1H NMR spectrum of 16 showed several signals at 0.08 and 0.84 ppm for ter-butyldimethylsylane fragment; at 1.58, 1.64, 2.20 and 2.36 ppm for methylene bound to both amino and alkyne groups; at 1.74, 2.04 and 2.28 ppm for methylene groups bound to both imino and cyclobutene ring; at 3.10, 3.18, 3.52 and 3.56 ppm for methylene groups bound to both imino and amino groups; at 4.52 ppm for amino groups; at 5.40 for proton of cyclobutene ring; at 7.70 and 8.20 ppm for imino groups. The 13C NMR spectra showed chemical shifts at 16.38, 24.92, 29.94 and 27.40 ppm for methylene groups bound to both imino and alkyne; at 25.22, 31.10 and 36.10 ppm for methylene groups bound to both imino and cyclobutene ring; at 40.54, 51.62-51.70 and 54.14-58.00 ppm for methylene bound to both imino and amino groups; 79.64 and 87.30 ppm for alkyne group; at 132.38 and 156.22 ppm for cyclobutene ring; at 152.24-156.18 and 169.34 ppm for imino groups. Finally, the presence of 16 was further confirmed from mass spectrum which showed a molecular ion at m/z: 732.58.
Other data showed several signals of the 1H NMR spectrum for 19 at 1.58, 1.66, 2.20 and 2.36 ppm for methylene groups bound to both imino and alkyne; at 2.06 and 2.30 ppm for methylene groups bound to both imino and cyclobutene ring; at 3.10, 3.18, 3.52 and 3.56 ppm for methylene groups bound to both amino and imino groups; at 4.60 ppm for amino groups; at 5.40 ppm for cyclobutene ring; at 7.70-8.20 ppm for imino groups. The 13C NMR spectra showed chemical shifts at 16.42, 24.90, 27.00 and 27.44 ppm for methylene groups bound to both alkyne and imino groups; at 25.22, 31.10 and 36.14 ppm for methylene groups bound to both imino and cyclobutene ring; at 40.56, 51.22-54.10 and 58.00 ppm for methylene groups bound to both imino and amino groups; at 79.60-87.28 for alkyne groups; at 132.40 and 156.28 for cyclobutene ring; at 152.26, 156.20, 162.68 and 169.28 ppm for imino groups. In addition, the presence of 19 was further confirmed from mass spectrum which showed a molecular ion at m/z: 836.68.
Finally, the 1H NMR spectrum of 21 shows signals at 1.40, 1.90 and 2.06-2.10 ppm for methylene groups bound to both imino and cyclobutene ring; at 1.76, 2.12 and 2.42 ppm for methylene bound to both alkyne and imino groups; at 3.10-3.52 ppm for methylene groups bound to both imino and amino groups; at 4.52 ppm for cyclobutene ring; at 8.10 ppm for imino groups. The 13C NMR spectra showed chemical shifts at 16.80, 27.46 and 30.30 ppm for methylene groups bound to both alkyne and imino groups; at 26.80, 27.82, 30.40 and 35.82 ppm for methylene groups bound to both imino and cyclobutene ring; at 40.50, 51.26-54.16 and 58.00 ppm for methylene groups bound to both amino and imino groups; at 86.46-87.32 ppm for alkyne groups; at 133.94-154.08 ppm for cyclobutene ring; at 154.50-167.70 ppm for imino groups. Finally, the presence of 21 was further confirmed from mass spectrum which showed a molecular ion at m/z: 760.61.
Removal of Silyl Fragment of 16 or 22 Via Hydrofluoric Acid to form 17 or 21 (Scheme 4 and 5)
There are several reagent for removal of silyl protecting groups from hydroxyl such as ammonium fluoride45 , tris(dimethylamino)sulfonium/difluorotrimethylsilicate46, hydro fluoric acid47 and others. In this study, hydrofluoric acid was used to removal of silyl-protecting group from hydroxyl of the compound 8 to form 9 (Scheme 5). The 1H NMR spectrum of 17 showed several signals at 1.58, 1.66, 2.20 and 2.36 ppm for methylene bound to both amino and alkyne groups; at 1.74, 2.04 and 2.28 ppm for methylene groups bound to both imino and cyclobutene ring; at 3.10, 3.18 and 3.52-3.56 ppm for methylene groups bound to both imino and amino groups; at 4.80 ppm for both hydroxyl and amino groups; at 5.40 for proton of cyclobutene ring; at 7.70 and 8.20 ppm for imino groups. The 13C NMR spectra showed chemical shifts at 16.38, 24.92, 26.94 and 27.40 ppm for methylene groups bound to both imino and alkyne; at 25.22, 31.10 and 36.10 ppm for methylene groups bound to both imino and cyclobutene ring; at 40.54, 51.62-58.00 ppm for methylene bound to both imino and amino groups; at 79.64-87.30 ppm for alkyne groups; at 132.38 and 156.22 ppm for cyclobutene ring; at 152.24-156.18 and 169.34 ppm for imino groups. Finally, the presence of 17 was further confirmed from mass spectrum which showed a molecular ion at m/z: 618.49.
 |
Scheme 5: Preparation of a di-diazacycloundeca-5,11-dien-steroid-5-amine derivative (20). Reaction of 14 with ethylenediamine (vi) to form an amino-steroid derivative (19). Click here to View scheme |
After, 19 was was reacted with Copper(II) chloride (vi) to form 20.
Other results showed several signals of the 1H NMR spectrum for 22 at 0.88 and 0.98 ppm for methyl bound to steroid nucleus; at 1.68 ppm for methyl group bound to imino group; at 1.40, 1.90 and 2.06-2.10 ppm for methylene groups bound to both imino and cyclobutene ring; at 1.76, 2.12 and 2.42 ppm for methylene bound to both alkyne and imino groups; at 3.10-3.56 ppm for methylene groups bound to both imino and amino groups; at 4.24 ppm for both hydroxyl and amino groups; at 6.00 ppm for cyclobutene ring; at 7.70-8.10 ppm for imino groups. The 13C NMR spectra showed chemical shifts at 16.80, 26.80, 27.46 and 35.82 ppm for methylene groups bound to both alkyne and imino groups; at 40.50, 51.24, 51.62-54.16 and 58.00 ppm for methylene groups bound to both imino and cyclobutene ring; at 40.50, 51.26-54.16 and 58.00 ppm for methylene groups bound to both amino and imino groups; at 86.46-87.32 ppm for alkyne groups; at 133.94-154.08 ppm for cyclobutene ring; at 154.50-162.70 ppm for imino groups. Finally, the presence of 22 was further confirmed from mass spectrum which showed a molecular ion at m/z: 646.52.
Synthesis of 1,4-diazacycloundeca-5,11-dien-steroid derivatives (Scheme 4, 5 and 6)
Thre are several reports shown the preparation of diazabicycle derivatives using different reagents such as piperidinone48, lithium hexamethyldisilazane49., sodium hydride50, azodicarboxylate51, di-tertbutyl-dicarbonate52, palladium on carbon53. In this study, the compounds 17, 19 or 22 were reacted with Copper (II) chloride to form the bicycle derivatives 18 or 20 or 23. The 1H NMR spectrum of 18 showed several signals at 1.74, 2.04 and 2.30 ppm for methylene groups bound to both imino and cyclobutene ring; at 3.10 and 3.52-3.58 ppm for methylene groups bound to both imino and amino groups; at 1.84, 1.88, 2.06, 2.32, 3.12 and 3.90-4.40 ppm for bicycle ring (1,4-diaza-cycloundeca-diene); at 5.40 ppm for cyclobutene ring; at 5.50 ppm for amino groups; at 6.70-8.20 ppm for imino groups. The 13C NMR spectra showed chemical shifts at 25.22, 31.10 and 36.10 ppm for methylene groups bound to both imino and cyclobutene ring; at 40.54, 45.87 and 52.72-58.00 ppm for methylene bound to both imino and amino groups; at 27.22, 28.88, 32.70-34.12, 43.60, 61.27-73.34 and 149.60 for bicycle ring (1,4-diaza-cycloundeca-diene); 132.38 and 156.28 ppm for cyclobutene ring; at 152.24, 159.88-169.34 ppm for imino groups. In addition, the presence of 18 was further confirmed from mass spectrum which showed a molecular ion at m/z: 618.49.
Other data showed several signals of the 1H NMR spectrum for 20 at 1.74, 2.04 and 2.29 ppm for methylene groups bound to both imino and cyclobutene ring; at 1.86-1.88, 2.06, 2.30, 2.80, 3.14 and 3.90-4.42 ppm for bicycle ring (1,4-diaza-cycloundeca-diene); at 3.10 and 3.46-3.58 ppm for methylene groups bound to both amino and imino groups; at 5.60 ppm for amino groups; at 5.40 ppm for cyclobutene ring; at 6.70-8.20 ppm for imino groups. The 13C NMR spectra showed chemical shifts at 25.24, 31.14 and 36.14 ppm for methylene groups bound to both imino and cyclobutene ring; at 26.30, 27.20, 28.84, 32.70-34.16, 43.60, 54.90, 61.26-73.40 and 149.60 ppm for bicycle ring (1,4-diaza-cycloundeca-diene); at 36.14, 40.56, 45.86, 52.22-52.76 and 58.00 ppm for methylene groups bound to both imino and amino groups; at 132.40 and 156.20 for cyclobutene ring; at 152.26, 159.90-169.28 ppm for imino groups. In addition, the presence of 20 was further confirmed from mass spectrum which showed a molecular ion at m/z: 836.68.
 |
Scheme 6: Synthesis of a diazacycloundeca-5,11-dien-steroid-5-amine derivative (23). Reaction of 15 with ethylenediamine (v) to form an amino-steroid derivative (21). Click here to View scheme |
After, 22 was prepared by removal of the ter-buthylsylane fragment of 21 with hydrofluoric acid (v). Finally, 22 was reacted with Copper(II) chloride (vi) to form 23.
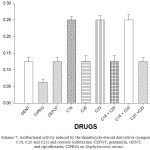 |
Scheme 7: Antibacterial activity induced by the diazabicyclo-steroid derivatives (compounds C18, C20 and C23) and controls (cefotaxime, CEFOT; gentamicin, GENT; and ciprofloxacin, CIPRO) on Staphylococcus aureus. Click here to View scheme |
The results showed that the bacterial growth of Staphylococcus aureus was inhibited with cefotaxime [MIC = 0.12 mg/ml], gentamicin [MIC = 0.12 mg/ml], ciprofloxacin [MIC = 0.06 mg], compound 18 [MIC = 0.25 mg/ml], compound 20 [MIC = 0.12 mg/ml], compound 23 [MIC = 0.25 mg/ml]. In addition, the growth of this microorganism was inhibited with the following mixtures; 18 + 20 [MIC = 0.12 mg/ml], 18 + 23 [MIC = 0.25 mg/ml] and 20 + 23 [MIC = 0.12 mg/ml].
MIC = Minimal inhibitory concentration.
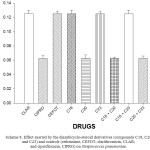 |
Scheme 8: Effect exerted by the diazabicyclo-steroid derivatives (compounds C18, C20 and C23) and controls (cefotaxime, CEFOT; clarithromicin, CLAR; and ciprofloxacin, CIPRO) on Streptococcus pneumoniae. Click here to View scheme |
The results showed that bacterial growth of Streptococcus pneumoniae was inhibited in the presence of cefotaxime [MIC = 0.12 mg/ml, 2.74), ciprofloxacin [MIC = 0.06 mg/ml], clarithromycin [MIC = 0.12 mg/ml] and compounds 18 [MIC = 0.12 mg/ml], 20 [MIC = 0.0.06 mg/ml] and 23 [MIC = 0.12 mg/ml]. Additionally, the bacterial growth of Streptococcus pneumoniae was inhibited with the following mixtures; 18 + 20 [MIC = 0.06 mg/ml], 18 + 23 [MIC = 0.12 mg/ml], 20 + 23 [MIC = 0.06 mg/ml]. MIC = Minimal inhibitory concentration.
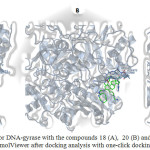 |
Schme 9: Site of binding for DNA-gyrase with the compounds 18 (A), 20 (B) and 23 (C). Visualized with GLmolViewer after docking analysis with one-click docking Click here to View scheme |
Finally, other data showed several signals of the 1H NMR spectrum for 23 at 1.40, 1.90 and 2.06-2.10 ppm for methylene groups bound to both imino and cyclobutene ring; at 1.86-1.88, 2.04, 2.30, 2.78, 3.12 and 3.96 ppm for bicycle ring (1,4-diaza-cycloundeca-diene); at 3.10-3.56 ppm for methylene groups bound to both imino and amino groups; at 4.80 ppm for both hydroxyl and amino groups; at 5.40 ppm for cyclobutene ring; at 6.70-8.10 ppm for imino groups. The 13C NMR spectra showed chemical shifts at 26.30, 27.20, 28.88, 34.12, 43.60, 54.88, 73.40 and 149.57 ppm for bicycle ring (1,4-diaza-cycloundeca-diene); at 26.80, 27.82, 30.40, 35.82, 54.88 and 154.50 ppm for methylene groups bound to both imino and cyclobutene ring; at 40.50, 45.82, 52.24 and 58.00 ppm for methylene groups bound to both amino and imino groups; at 133.94-154.08 ppm for cyclobutene ring; at 159.90-162.70 ppm for imino groups. Finally, the presence of 23 was further confirmed from mass spectrum which showed a molecular ion at m/z: 646.52.
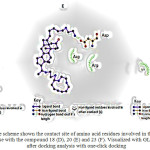 |
Scheme 10: The scheme shown the contact site of amino acid residues involved in the interaction of DNA-gyrase with the compound 18 (D), 20 (E) and 23 (F). Visualized with GL mol Viewer after docking analysis with one-click docking Click here to View scheme |
Evaluation of Biological Activity for Steroid Derivatives
In this study the antibacterial activity of some steroid derivatives (compounds 1-23) on Staphylococcus aureus and Streptococcus pneumoniae was evaluated by means of the dilution method and the minimum inhibitory concentration [MIC], using gentamicin, clarithromycin, ciprofloxacin and cefotaxime as control. The results showed that the bacterial growth of Staphylococcus aureus was inhibited with cefotaxime [MIC = 0.12 mg/ml], gentamicin [MIC = 0.12 mg/ml], ciprofloxacin [MIC = 0.06 mg], compound 18 [MIC = 0.25 mg/ml], compound 20 [MIC = 0.12 mg/ml], compound 23 [MIC = 0.25 mg/ml]. In addition, the growth of this microorganism was inhibited with the following mixtures; 18 + 20 [MIC = 0.12 mg/ml], 18 + 23 [MIC = 0.25 mg/ml] and 20 + 23 [MIC = 0.12 mg/ml].
Other results showed that bacterial growth of Streptococcus pneumoniae was inhibited in the presence of cefotaxime [MIC = 0.12 mg/ml, 2.74), ciprofloxacin [MIC = 0.06 mg/ml], clarithromycin [MIC = 0.12 mg/ml] and compounds 18 [MIC = 0.12 mg/ml], compound 17 [MIC = 0.06 mg/ml], 20 [MIC = 0.12 mg/ml] and 23 [MIC = 0.12 mg/ml]. Finally, the bacterial growth of Streptococcus pneumoniae was inhibited with the following mixtures; 18 + 20 [MIC = 0.06 mg/ml], 18 + 23 [MIC = 0.12 mg/ml], 20 + 23 [MIC = 0.06 mg/ml]. All these data indicate that; i) compounds 18, 20 and 22 has different antibacterial potency for Staphylococcus aureus and Streptococcus pneumoniae in comparison with gentamicin (an inhibitor of protein synthesis)54, and clarithromycin (protein synthesis inhibitor) [54], this phenomenon may be attributed mainly to the different molecular mechanism involved and the characteristic chemical structure of the compounds studied; ii) the compound 20 exerts greater antibacterial activity against Staphylococcus aureus and Streptococcus pneumoniae compared with the compounds 18 and 23; iv) the antibacterial effect of 20 was similar to cycprofloxacin. This phenomenon could depend on the interaction of two diazabicyclo rings involved in the chemical structure of 20 with some cellular structure involved in the microorganisms studied. This hypothesis can be availed by some reports which indicate that antibacterial activity of cycprofloxacin is via interaction with DNA gyrase55; v) finally, the different mixtures evaluated in this study do not increase the antibacterial activity compared with the compound 20 against Staphylococcus aureus and Streptococcus pneumoniae.
Docking Evaluation
In order, to evaluate the possibility that the compound 20 could interact with DNA gyrase (PDB ID:2xcr)56 in this study a molecular docking model (serverdoking)57 was used58,59. Theoretical results indicate that hydrogen-interaction between compound 20 and DNA gyrase (Figure 11, 12 and Table 1) involves several amino acid residues such as Leu704, Asn705, Met780, Cys784, Met749, Leu762,Phe764, Ser778 and Met787. In addition, other theoretical results showed the decomposed interaction energies (Kcal/mol) between the compound 20 and the amino acid residues from DNA gyrase (table 2). All these data suggest that the interaction of compound 20 with DNA gyrase is conditioned by their physicochemical properties.
Table 1: Aminoacid residues involved between the interaction of diazabicyclo-steroid derivatives (18, 20 and 23) with the DNA-gyrase surface.
|
Compounds I—————————–I Interactions* 18 20 23 |
|||
| Hidrogen bonds |
Asp470 |
|
Arg470 |
| Polar |
Asp470 Arg471 |
Arg471 |
Arg468 Arg470 |
| Others |
Lys466 Asp470 Arg471 |
Arg468 Asp470 Arg471 |
Arg468 Arg477 |
*aminoacids residues
Table 2: Descompesed interaction energies (Kcal/mol) involved between the diazabicyclo-steroid derivatives (18, 20 and 23) and DNA-gyrase surface.
|
Compounds I—————————————-I Interactions* 18 20 23
|
|||
| Hidrogen bonds |
Asp470 (-0.3491) |
|
Arg470 (0.0708) |
| Polar |
Arg471 (-1.4456) |
Arg471 (-1.6256) |
Arg468 (-1.2042) |
| Others |
Arg468 (-1.0132) Lys466 (-0.9578) |
Arg468 (-2.2648) Asp470 (-0.1188) |
Arg471 (-0.8197) |
*aminoacids residues
Evaluation Lipophilicity Degree of Compounds 18, 20 and 23
In order to delineate the structural chemical requirements of compounds 18, 20 and 23; other parameters such as the logP and πdescriptors were calculated, with the purpose to know if there are differences in its lipophilicity degree60. There are studies which indicate that logP estimates the logarithmic octanol-water partition coefficient; therefore the logP represents the lipophilic effects of a molecule which includes the sum of the lipophilic contributions of the parent molecule and its substituent61. The difference between the substituted and unsubstituted logP values is conditioned by the π value for the particular substituent. Hammett showed that π values measure the free energy change caused by particular substituent to relate to biological activity62, 63.
Table 3: Estimated docking parameters involved between the interaction of diazabicyclo-steroid derivatives (18, 20 and 23) with the DNA-gyrase surface.
|
Compounds |
Est. free energy of binding (kcla/mol) |
WW + H bond + desolv energy |
Electrostatic energy |
Interaction surface |
Total |
|
18 |
-3.80 |
-3.80 |
-0.87 |
498.002 |
-4.67 |
|
20 |
2.34 |
-3.55 |
0.53 |
543.180 |
-3.02 |
|
23 |
-0.33 |
-2.70 |
-1.64 |
397.296 |
-4.33 |
Analyzing this data, in this study the log P and π parameters were calculated by the method proposed by Mannhold and Waterbeemd64. The results (Table 4) showed an increase in both logP and π values of 20 with respect to the compounds 18 and 23 which is mainly conditioned by the contribution of all the substituent atoms involved in the chemical structure of compound 20. This phenomenon conditions the interaction of compound 20 with the DNA gyrase and subsequently decreases the bacterial growth of Staphylococcus aureus and Streptococcus pneumoniae bacteria.
Table 4. Physicochemical parameters [log P (log Kow), and π] of compounds 18, 20 and 23.
| Compound 18 | -CH3 [aliphatic carbon] | 1.0946 |
| -CH2- [aliphatic carbon] | 10.3131 | |
| -CH [aliphatic carbon] | 2.5298 | |
| C [aliphatic carbon- No H, not tert] | 0.9723 | |
| =CH- or =C< [olefinic carbon] | 1.5344 | |
| -OH [hydroxyl, aliphatic attach] | -1.4086 | |
| -NH2 [aliphatic attach] | -1.4148 | |
| -NH [aliphatic attach] | -2.9924 | |
| -tert Carbon [3 or more carbon attach] | 0.8028 | |
| -N=C [aliphatic attach] | -0.003 | |
| Fused aliphatic ring unit correction | -2.0526 | |
| >C=N-C [cyclic-type imine, ali carbon attach] | -1.55 | |
| C=C(-N-)-N- correction | 0.6 | |
| -CH=N-C [linear imine] correction | -1.2 | |
| Equation Constant | 0.229 | |
| π | 2.2146 | |
| Compound 20 | Log Kow | 7.4546 |
| -CH3 [aliphatic carbon] | 1.6419 | |
| -CH2- [aliphatic carbon] | 14.2419 | |
| -CH [aliphatic carbon] | 2.8912 | |
| C [aliphatic carbon- No H, not tert] | 1.9446 | |
| =CH- or =C< [olefinic carbon] | 2.3016 | |
| -NH2 [aliphatic attach] | -1.4148 | |
| -NH [aliphatic attach] | -5.9848 | |
| -tert Carbon [3 or more carbon attach] | 0.8028 | |
| -N=C [aliphatic attach] | -0.005 | |
| Fused aliphatic ring unit correction | -2.0526 | |
| >C=N-C [cyclic-type imine, ali carbon attach] | -15500 | |
| C=C)-N-)-N- correction | 1.2 | |
| -CH=N-C [linear imine] correction | -1.2 | |
| Equation Constant | 0.229 | |
| Compound 23 | π | 4.4358 |
| Log Kow | 13.0458 | |
| -CH3 [aliphatic carbon] | 1.6419 | |
| -CH2- [aliphatic carbon] | 10.8042 | |
| -CH [aliphatic carbon] | 2.8912 | |
| C [aliphatic carbon- No H, not tert] | 0.9723 | |
| =CH- or =C< [olefinic carbon] | 1.5344 | |
| -OH [hydroxyl, aliphatic attach] | -1.4086 | |
| -NH2 [aliphatic attach] | -1.4148 | |
| -NH [aliphatic attach] | -2.9924 | |
| -tert Carbon [3 or more carbon attach] | 0.8028 | |
| -N=C [aliphatic attach] | -0.003 | |
| Fused aliphatic ring unit correction | -2.0526 | |
| C=C (-N-)-N correction | 0.6 | |
| -CH=N-C [linear imine] correction | -1.2 | |
| Equation Constant | 0.229 | |
| π | 2.71 | |
| LogKow | 10.4 |
Conclusions
The diazabicyclo-steroid derivative (compound 20) is a particularly interesting drug, because its antibacterial activity exerted against Staphylococcus aureus and Streptococcus pneumoniae involves a molecular mechanism different in comparison with other drugs; this phenomenon may constitute a novel therapy for infectious diseases.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Pinner, R.; Teutsch, A.; Simonsen, L.; J. Am. Med. Assoc. 1996, 275, 189-193.
CrossRef - Crossley, K.; Peterson, P.; Clin. Infect. Dis. 1996, 22, 209-214.
CrossRef - Chambers, H.; Emerg. Infect. Dis. 2001, 7, 178-182.
CrossRef - Bogaert, D.; De-Groot, R.; Hermans, P.; Lancet Infect. Dis. 2004, 4, 144-154.
CrossRef - Yoo, B.; Triller, D.; Yong, C.; Lodise, T.; Ann. Pharmacother. 2004, 38, 1226-1235.
CrossRef - Killgore, M.; March, K.; Guglielmo, B.; Risk.; Ann. Pharmacother. 2004, 38, 1148-1152.
CrossRef - Hackbarth, C.; Chambers, H.; Antimicrob. Agents Chemother. 1989, 33, 991-994.
CrossRef - Maguire, G.; Arthur, A.; Boustead, P.; Dwyer, B.; Currie, B.; J. Hosp. Infect. 1998, 38, 273-281.
CrossRef - Parthiban, P.; Rathika, P.; Ramkumar, V.; Mo, S.; Tae, Y.; Bioorg. Med. Chem. Lett. 2010, 20, 1642-1647.
CrossRef - Oh, C.; Dong, H.; Cho, H.; Park, S.; Hong, J.; Baek, D.; Cho, J.; Arch. Pharm. Med. Chem. 2002, 5, 200-206.
CrossRef - Balaji, G.; Rajesh, K.; Janardhan, R.; Vijayakumar, V.; Res. Chem. Intern. 2015, 41, 6497-6509.
CrossRef - Sedavkina, V.; Morozova, N.; Rechinskaya, A.; Kulikova, L.; Pharm. Chem J. 1974, 8, 21-24.
CrossRef - Burakova, E.; Saranina, I.; Tikunova, N.; Silnikov, N.; Russian Chem. Bull. 2015, 64, 1400-1405.
CrossRef - Arutyunyan, R.; Paronikyan, G.; Saakyan, A.; Arutyunyan, K.; Pharm. Chem. J. 2008, 42, 18-22.
CrossRef - Ponnuswamy, S.; Pushpalatha, S.; Akila, A.; Raghuvarman, B.; Aravindhan, S.; J. Mol. Struct. 2016, 1125, 453-463.
CrossRef - Aldridge, K.; Ashcraft, D.; Antimicrob. Agents Chemother. 1997, 41, 709-711.
CrossRef - Blackman, S.; Balti, R.; J. Org. Chem. 1961, 26, 2750-2755.
CrossRef - Brueggemann, A.; Kugler, A.; Doern, G.; Antimicrob. Agents Chemother. 1997, 41, 1594-1597.
CrossRef - Ikee, Y.; Hashimoto, K.; Nakashima, M.; Hayashi, K.; Sano, M.;, Bioorg. Med. Chem. Lett. 2007, 17, 942-945.
CrossRef - Figueroa-Valverde. L.; Díaz-Cedillo, F.; García-Cervera, E.; Pool-Gómez, E.; López-Ramos, M.; Rosas-Nexticapa, M.; Hau-Heredia, L.; Sarabia-Alcocer, B.; Steroids. 2015, 93, 8-15.
- Figueroa-Valverde, L.; García-Cervera, E.; Díaz-Cedillo, F.; Hau-Heredia, L.; Rosas-Nexticapa, M.; Pool-Gómez, E.; López-Ramos, M.; Camacho-Luis, A.; Asian J. Chem. 2016, 28, 2357-2364.
CrossRef - Halgren, T.; J. Comput. Chem. 1999, 20, 720-729.
CrossRef - Nahta, R.; Yu, D.; Hung, M.; Hortobagyi, G.; Esteva, F.; Nature Clin. Pract. Oncol. 2006, 3, 269-280
CrossRef - Bièche, I.; Murcia, G.; Lidereau, R.; Clinical Cancer Research. 1996, 2, 1163-1167
- Morris, M.; Goodsell, M.; Hallyday, D.; Huey, R.; J. Comput. Chem. 1999, 19, 1639-1662.
CrossRef - Solis, F. Mathem. Meth. Oper. Res. 1981, 1, 19-30.
CrossRef - Hocht, C.; Opezzo, J.; Gorzalczany, S.; Bramuglia, G.; Tiara, C.; Rev. Arg. Cardiol. 1999, 67, 769-773.
- Figueroa, L.; Diaz, F.; Ceballos G.; Lopez, M.; Maldonado, G.; Camacho, A.; J. Argentine Chem. Soc. 2008, 96, 87-100
- Chao, K.; Rayabarapu, D.; Wang, C.; Cheng, C.; J. Org. Chem. 2001, 66, 8804-8810.
CrossRef - Zhao, L.; Zhang, L.; Fang, D.; Organometallics. 2016, 35, 3577-3586.
CrossRef - Nishimura, A.; Ohashi, M.; Ogoshi, M.; J. Am. Chem. Soc. 2012, 134, 15692-15695.
CrossRef - Goodreid, J.; Villeneuve, K.; Carlson, E.; Tam, W.; J. Org. Chem. 2014, 9, 10002-10012.
CrossRef - Uppiah, D.; Bhowon, D.; Jhaumeer, M.; E-J. Chem. 2009, l, S195-200.
- Figueroa-Valverde, L.; Díaz-Cedillo, F.; García-Cervera, E.; Rosas-Nexticapan, M.; Ramos-López, M.; Asian J. Chem. 2013, 5, 6724-6726.
- Hania, M.; E-J. Chem. 2009, 6, 629-632.
CrossRef - Josephsohn, N.; Carswell, E.; Snapper, M.; Hoveyda, A.; Org. Lett. 2005, 7, 2711-2713.
CrossRef - Zhang, H.; Bonaga, L.; Ye, H.; Derian, C.; Damiano, B.; Maryanoff, B.; Bioorg. Med. Chem. Lett. 2007, 17, 2863-2868.
CrossRef - Reza, A.; Nasreesfahani, Z.; Ruoho, A.; Org. Prep. Proced. Int. 2008, 40, 385-391.
CrossRef - Fisher, G.; Lee, L.; Klettke, F.; Int. J. Rapid. Comm. Syn. Org. Chem. 1994, 24, 1541-1546.
- Vedejs, E.; Fuchs P. Improved aldehyde synthesis from 1,3-dithianes. J. Org. Chem. 1971, 36, 366-367.
CrossRef - Billard, F.; Robiette, R.; Pospíšil, J.; J. Org. Chem. 2012, 77, 6358-6364.
CrossRef - Holum, J.; J.Org. Chem. 1961, 26, 4814-4816.
CrossRef - Tokunaga, M.; Suzuki, T.; Koga, N.; Fukushima, T.; Horiuchi, A.; Wakatsuki, Y.; J. Am. Chem. Soc. 2001, 123, 11917-11924.
CrossRef - Zhang, W.; Robins, M.; Tetrahedron Lett. 1992;33:1177-80.
CrossRef - Scheidt, K.; Chen, H.; Follows, B.; Chemler, S.; Coffey, D.; Roush, W.; J. Org. Chem. 1998, 63, 6436-6437.
CrossRef - Newton, R.; Reynolds, D.; Finch, M.; Kelly, D.; Roberts, S. Tetrahedron Lett. 1979, 20, 3981-3982.
CrossRef - Siener, T.; Cambareri, A.; Kuhl, U.; Englberger, W.; Haurand, M.; Kögel, B.; Holzgrabe, U.; J. Med. Chem. 2000, 43, 3746-3751.
CrossRef - Weigl, M.; Wünsch, B.; Org. Lett. 2000, 2, 1177-1179.
CrossRef - Manetti, D.; Ghelardini, C.; Bartolini, A.; Bellucci, C.; Dei, S.; Galeotti, N.; Gualtieri, F.; Romanelli, M.; Scapecchi, S.; Teodori, E.; J. Med. Chem. 2000, 43, 1969-1974.
CrossRef - Khrizman, A.; Slack, R.; Remsing, R.; Little, S.; Yardley, V.; Moyna, G.; Arch. Pharm. Chem. Life. Sci. 2007, 340, 569- 576.
CrossRef - Barlocco, D.; Cignarella, G.; Tondi, D.; Vianello, P.; J. Med. Chem. 1998, 41, 674-681.
CrossRef - Kinney, W.; Abou-Gharbia, M.; Garrison, D.; Schmid, J.; Kowal, D.; Bramlett, D.; Miller, T.; Tasse, R.; J. Med. Chem. 1998, 41, 236-246.
CrossRef - Lin, J.; Connelly, M.; Amolo, C.; Otani, S.; Yaver, D.; Antimicrob. Agents Chemother. 2005, 49, 1915-1926.
CrossRef - Tateda, K.; Ishii, Y.; Matsumoto, T.; Furuya, N.; Antimicrob. Agents Chemother. 1996, 40, 2271-2275.
CrossRef - Pan, X.; Ambler, J.; Mehtar, S.; Fisher, L.; Antimicrob. Agents Chemother. 1996, 40, 2321-2316.
CrossRef - Calleja, C.; Pascussi, J.; Mani, J.; Maurel, P.; Vilarem, M.; Nat. Med. 1998, 4, 92-96.
CrossRef - Liu, R.; Perez, J.; Liang, D.; Saven, J.; Anesth. Analg. 2012, 114, 947-955.
CrossRef - Rosales, M.; Correa, J.; Expert. Opinion Drug. Dis. 2015, 10, 213-219.
CrossRef - Askew, E.; Gampe, R.; Stanley, T.; Faggart, J.; Wilson, E.; J. Biol. Chem. 2007, 282, 25801-25816.
CrossRef - Leo, A.; Jow, J.; Silipo, C.; J. Med. Chem. 1975, 18, 865-868.
CrossRef - Leo, A.; Perspect. Drug Discov. Design. 2000, 18, 19-38.
CrossRef - Hansch, C.; Leo, A.; Taft, R.;. Chem. Rev. 1991, 91, 165-195.
CrossRef - Figueroa-Valverde, L.; Díaz-Cedillo, F.; Camacho-Luis, A.; López-Ramos, M.; Garcia-Cervera, E.; Monatsh. Chem. 2010, 141, 373-380.
CrossRef - Mannhold, R.; Waterbeemd, H.; J. Comput. Aided Mol. Design. 2001, 15, 337-354.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









