Thin Double Wall Boron Nitride Nanotube: Nano-Cylindrical Capacitor
Marzieh Sadat Madani, Majid Monajjemi, Hossein Aghaei and Masoud Giahi
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: m_monajjemi@srbiau.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/330320
Experimental results have shown that a small single wall carbon Nano tubes (SWCNTs) can be usually found inside “multi-walled” (MWCNTs). In this work it has been reported the stabilities and electronic structure of the single wall boron nitride Nano-tube (SWBNNTs) inside SWBNNTs. It has been shown that the gap energies and that energy of BNNTs are strongly dependent on their diameters or chirality. When this kind of BNNTs are inserted in the larger one of SWBNNTs, the gap energies of the double walled (DWBNNTs ) would even be much decreased due to the “coupled effect” of “wall buckling” difference and inter-wall π-π* “hybridization”. DWBNNTs are used for a theoretical study of a cylindrical molecular capacitor, including an inner cylinder with a positive charge distribution and an outer cylinder with a negative charge distribution. Due to their semiconductor characteristic and dielectric functionalities of SWBNNTs, DWBNNTs can be used as a cylindrical capacitor for the electronic devices.
KEYWORDS:Single-wall BN-nanotubes; nano-cylindrical capacitors
Download this article as:| Copy the following to cite this article: Madani M. S, Monajjemi M, Aghaei H, Giahi M. Thin Double Wall Boron Nitride Nanotube: Nano-Cylindrical Capacitor. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Madani M. S, Monajjemi M, Aghaei H, Giahi M. Thin Double Wall Boron Nitride Nanotube: Nano-Cylindrical Capacitor. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33444 |
Introduction
NSCCE or “Nanometer-scale capacitive charging effect” currently is familiar, e.g., the “coulomb blockade phenomenon” in the quantum dots1,2 . However, they are arduous for resolving the detailed radial charges repartition within the nanostructures. Using SWBNNTs @ SWBNNTs double wall tubes; it has been discussed the “radial” charge distributions of multilayered molecular capacitors.
Designing and control of Nano-tubes diameters are requirement to develop Nano-tube growth method. Nano-tubes with the diameters of less than one nanometer provide the ideal Nano-spaces in X-dimension3. A few years ago, it has been suggested which the sizes of the catalysts used in the metal catalyze CVD (chemical vapor deposition) can explain the diameter of grown carbon nanotube4.This opinion has been confirmed by the seeing that a catalytic particle in the end of chemical vapor deposition grown nanotube has size proportional with the Nano-tubes diameters5.
Chin Li Cheung, illustrate clearly the concepts of different sizes nano cluster catalysts which they can be used for controlling the structures and diameters of “CVD” grown nanotubes6. Small-diameter carbon nanotubes indicate to many exotic properties such as an-isotropic optical absorption spectra7 and superconductivities emanate from a “Pearle” distortion8,9. This finding has stimulated much fondness in studies of a small nanotube in both theoretically and experimentally10-14 . BN-Nano-tubes (BNNTs), which firstly synthesized and predicted through Chopra work and Rubio respectively15,16, has a unique structural syllogism to carbon Nano-tubes but, contrary to the C-N-T being semiconductor or metallic depending on their chirality16. BNNT is usually can be used as an insulator regardless of its diameter and helicity or the number of walls15-17.
Experimental result has shown that a small SWNT is usually found inside a multi-walled CNT11-13. Therefore, there are strong incentive for studding in details the stabilities and interaction of narrow BNNT inside a bigger one in viewpoint of diameter, which makes easier for understanding the experimental result. Furthermore, the studies of double-walled boron nitride Nano-tubes (DBNNTs) have displayed interesting variations in its electronic properties comparing with those of free-standing part of BNNTs18. So it is also significant for seeing the interaction energies associated and inter wall coupling behavior with the narrow BNNTs17,18.
B-N nanotube possesses grate band gap around 6 eV regardless of diameters, chirality, electronic properties and the number of walls15, 19. Furthermore, they are stable in view-point of mechanical and chemical structures20. Therefore, narrow single-wall BNNT can widely be applies as an ideal Nano-tube for the Nano sciences for producing suitable material such as; capacitor, atomic wire, and semiconductor21, 22. In our study the SWBNNs are special material as an insulator for producing Nano-cylindrical capacitors22.
Ryo Nakanishi reported an important synthesis method of a narrow SWBNNT having uniform distribution of diameter around 0.7 0.1 nm1. Their strategies to synthesize thin BNNTs are for combining the Nano-template reaction using Single wall carbon Nano-tubes which has developed in the past ten years23. In their systems, precursor molecules including Ammonia Borane Complexes or ABC, including boron and nitrogen were en-capsulated first in the single wall carbon tubes followed by suitable thermal decomposition-fusion22 reaction inside the SWCNTs22,23.
It has been used arc-grown SWCNTs24 with distribution (diameter) around 1.4 nm24 as a model for synthesizing narrow SWBNNT22,24. Those distributions of SWCNT-models are essential for realizing the diameter selective synthesizing of BNNTs.
In this investigation, the systems have been simulated based on the various distribution diameters of SWBNNTs @SWBNNTs corresponding the experimental results of Ryo Nakanishi results1 .So we have started for answering to some questions for the mechanism of the radial charge distributions on the inner and outer electrodes, band gapes, potential difference23 between two layers of the Nano cylindrical24 capacitor and the capacitance of our system when the inner tubes are semiconducting25, and the others are metallic26.
Gugang Chen in the studies of doped double-walled carbon nanotubes26 exhibited the Resonant- Raman-Scattering (RRS) from the phonons26 on each carbon shell determines the radial charges distributions26. The self-consistent including tight-binding model (SCTB) confirms25 the observed molecular faraday-cage effects, so most of the charges reside on the outer-walls, even when these walls were originally26 semiconducting and the inner-walls were metallic24-26.
Those systems have been modeled as three-layer cylindrical capacitor within bromine anion forming the shell (around the outer nanotubes26). The total energies contain 3 terms including the inner tube, outer tube and band structures of the electrostatic energies “ for the three-layer charge distributions:
![]()
The signs “ i ” and “ k ” label the wave vector and occupied band for the outer or inner tube26.
They assumed that the surpluses charges on both shells are distributed in the infinitely thin-walls at the nuclear radiuses of those shell26, 27. The resulting electrostatic energies of the triple-walled capacitors are:
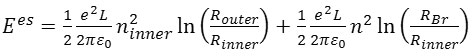
where(ε0) is the permittivity of free space27, L is the unit-cell length of the outer tubes and (n) is the linear densities of surpluses holes for the inner tubes.
There are two physical properties acts in concert27 for isolating most of the holes into the outer nanotubes. (1): the gap band of the thinner diameter tubes towards the larger one, so they empty last28. (2) The cylindrical-geometry rather raises the electrostatics potentials in the inner tubes27. Lonely the charges on the inner tubes in the chirality of Zigzag (n, 0) of BNNTs are anticipated to have direct band gaps27. On the other side the armchair (n, n) of BNNTs will have indirect band gaps27. Because of its large band gap around 5 eV, experiments28 using BNNT as the conduction-channel29 for field effect transistors29 or FETs showed that BNNT allowed transport through only the valence band28. The other important features about the band gaps of BNNTs are that they are tunable by doping with carbons29, radial deformation30, or by applying the transverses electric fields through the BNNTs so-called giant-stark-effect31-33 .
Theoretical band structure calculation suggested that “SWBNNT” can either be n-type or p-type semiconductor by controlling the composition of carbon into “SWBNNTs”. Carbon impurities on a boron site result in electron carriers while on a nitrogen site result in hole carriers34.
In this study it has been exhibited that the piezoelectricity34 for SWBNNTs causes for increasing the capacities of SWBNNTs @ SWBNNTs capacitor comparing to SWCNTs @ SWCNTs. This phenomenon35 originated from the deformation effects due to the tumbling of the planar hexagonal boron nitride network to produce tubular structures35. It has been exhibited by Nakhmanson that BNNT could be excellent piezoelectric systems36. As instant, piezoelectric constants for variant zigzags of SWBNNT were found for increasing along with the decreaseing of the radius in several BNNTs36. Experimentally37, Bai has exhabited that under in situ elastic bending deformation36 or EBD at room temperature high-resolution-transmission-electron- microscope36,37, a normally electrically insulating37 MW BNNT may transform to a semiconductor37.
K. Uchida et al38-39 in a discussion of quantum effect in the cylindrical carbon nanotubes capacitor exhibited that the distributions of the accumulated charges in the inner tubes are quantum mechanically37 spilled outward, while that in the outer tubes are penetrating inwards38. They have shown the reflecting those charges spills, the electrostatic capacitance of the systems are larger than what would be expected from the classical theories38.
Finally, they have shown that the capacitance exhibit two principal quantum effects, (1): the capacitance shows a large bias dependence38, reflecting the densities of states of the carbon Nano tube electrodes. (2): the capacitances are enhanced according to a quantum mechanical spill of the stored electrons density from the tubes walls of the CNTs36-38.
Based on our previous works40-61, we simulated our model in viewpoint of different band gap energies via considering the single wall boron nitrides as both inner and outer tubes with variant diameters and chirality in the ranges of (6.0 <d<8.0 ) and (11.0 <d< 16.0 ) for inner and outer tubes respectively.
Theoretical Background
Carbon nanotube is thin seamless graphitic cylinders39, which exhibit an unusual combination of the Nano-meter size diameter and Milli-meter size length39. These topologies, are included with the absence of defects38 on the macroscopic scales62, yields to uncommon electronic properties of individual62,63 SWCNTs that depends on their diameters and chirality63, can be either63 insulating, metallic or semiconducting64,65.
Consider a cylindrical capacitor of length “di”, inner radius “Rinn”, outer radius “Rout”, and with charges Q =di.qλ which qλ is the charges per unit length (magnitude) on each cylinders (Scheme1). Assume “di”, >> “Rinn”, or “Rout”, and Neglect fringing and electric fields between cylinders: use Gauss’ law
![]()
and electric potentials between cylinders: use
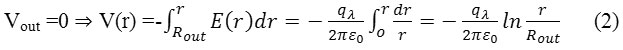
and
![]()
and capacitances for cylindrical geometries are:
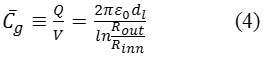
which “K” is the dielectric constant of the system65.
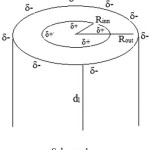 |
Scheme 1 Click here to View scheme |
![]()
is positive quantities because
![]()
is a positive quantities these because outer layers are at a higher potential than the inner layers. In our systems the calculation of the nano-cylindrical capacitors can be obtained from the electrical potential
ø(r,z)
in the spaces between two coaxial cylinders of radii Rinn and Rout and finite length di in the z direction,
0 ≤ z ≤ di.
We supposed the geometrical capacitances in our systems are a function of di, Rinn Rout
or
Cg = F(di,Rinn, Rout).
Bilalbegovi66-68with a molecular dynamic simulation and extension series based on Bessel functions69 has modified and discussed that capacitance around the shorter coaxial cylinders of radios69. So the capacitance in our model can be calculated via
![]()
in the finite Nano-meter scales cylindrical-capacitor based on the classical electrodynamic85. For calculation the capacitance for the eq.4 and eq.5 for
![]()
the potential difference applied between two cylindrical plates V=V(inn)-V(out) has been calculated by pop = chelpG commands76.
Computational Details
Calculations were accomplished using GAMESS-US packages72. In this work, it has been mainly focused for optimization of each tube with DFT methods73-77 consist of the m06 and m06-L74. The m062x74, m06-L74, and m06-HF74 are a unique Meta hybrid74 DFT functional with a good correspondence in non-bonded78 calculations and are useful for calculating the energies of the distances between two coaxial cylinders of radii and in the cylindrical capacitor73. The Perdew, Burkeand Ernzerhof (PBE)75 exchange correlation75 (XC) functional74,75 of the generalized-gradient-approximations95 (GGA) are adopted. The lattice constant has been optimized for the atomic coordinate and has done through the minimization of the total energies. For geometries optimizations, all the internal coordinates were relaxed until the Hellmann-Feynman-forces74 was less than 0.005 angstrom.
At each inter tube configurations, a single point calculation is carried out and the total energies are recorded. The resulting sliding rotation energy surface is used for fixing our model in a better position.
We employed DFT theories with the van der Waals DFT for modeling the exchange-correlation energies of SBNNTs and SWCNs76. The {ζ-basis set} with polarization76 orbital was used for single wall tube76.
For non-covalent approaches, DFT methods disable for describing van der Waals73,77 . The other functional are correctly insufficient for showing the correlation and exchange energies in non-bonded medium-ranges distances. Furthermore, recent study has illustrated that the medium-range exchange78,79 energies leads to the large systematic errors78 in the prediction of molecular propertie79.
We further calculated the interaction energies between two coaxial cylinders of radii “Rinn” and “Rout”
for SBNNTs and SWCNs in the structures. The dielectric permittivities as function of dielectric size were determined via Abinito calculation78-79. The interaction energies for capacitor were calculated via an extendedhuckel method in all items according to the eq.6.
![]()
Where the “ΔEs” is the stability energy of capacitor.
The charge transfer and electrostatic potential-derived also calculated using the “Merz-Kollman-Singh80, Chelp81, ChelpG82 and MESP83-84.
The MESPs are calculated and distributed at large number of grid points84 in a cube regularly.
The representative85 atomic charges86 would be computed as averages values over a few molecular conformations for the molecules86,87.
The electron densities Both of & Laplacian88 and Gradient norms89, value of orbital wave function90, electron spin densities88, localization function (ELF)89, localized orbital locator90, electrostatic potential88 from nuclear- atomic-charges, electron total electrostatic potential (ESP)90, and the exchange-correlation density, correlation hole and correlation factor, Average local ionization energy using the Multifunctional-Wave-function Analyzer88-90.
Result and Discussion
We first consider the h-BN sheet and 3D BN tubes in various diameters and chirality where the (5, 5) @ (8, 8) structure is found as a stable form compared to other forms. The stability depends on the distance between inner radius “Rinn”, and outer radius “Rout in one hand and the chirality on other hand Table1. The minimum energies are calculated based on eq.9 in terms of the total energy of the optimized structures and are listed in Table1.
The differences in the band structure and Fermi88 level energy of different tubes have been calculated. Furthermore, we have presented the number of states in unit energy interval through density of states (DOS) (Fig.1). DOS88 was plotted as a curved map and we have considered those graphs as a tool for analyzing the nature of electronic structure in our systems88,89. The original total DOS (TDOS)89 of our system was calculated based on References {108-110}108-110 in Figs. 2, 3, 4 and 5.
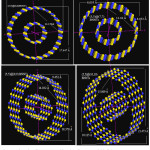 |
Figure 1: Dimension of MWBNNTs with various diameters and chiralities |
 |
Figure 2: TDOS, PDOS, OPDOS versus energy for (5,5)@(8,8) BNNTs Click here to View figure |
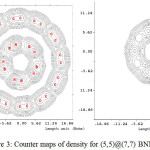 |
Figure 3: Counter maps of density for (5,5)@(7,7) BNNTs Click here to View figure |
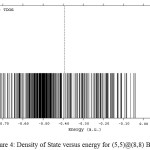 |
Figure 4: Density of State versus energy for (5,5)@(8,8) BNNTs Click here to View figure |
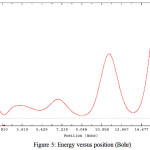 |
Figure 5: Energy versus position (Bohr) Click here to View figure |
The interaction energies for capacitors were calculated in all items according to the eq. 6
ΔEs(eV) = (Ec- {(2EDopant-G + Eh-Bn)} +EBSSE
Where the “ΔES” is the stability energy of capacitor.
The wall buckling has been defined as the differences between the mean radiuses91 of the cylinders consisting of the B and N atoms. It has been shown that the buckling rapidly increases92 with decreasing of the tube diameter91 and are independent of the chirality92,93.
The (5,5) SWBNNTs in the form of inner cylindrical are semiconductors due to the existence of an energy gap in the range of (7.55-9.67) KJ/mol (Table1) which are between the valence band and the conductor93 band. Those armchair tubes have a direct band gap, similar to the BN structure94.
The projected local density of states (PDOS) of B and N are plotted in Fig.2 together with the total DOS for comparison. The data for interaction energy shows that the (5, 5) @ (8, 8) DWBNNTs have a stable form comparing to other systems which yields a suitable charge transfer for the Nano capacitor92,93.
The calculated values of charge transfer for (5, 5)B-N-NTs @ (7, 7)B-N-NTs, (5,5)B-N-NTs @ (8, 8)B-N-NTs and (5, 5)B-N-NTs @ (9, 9)B-N-NTs and (5, 5)B-N-NTs @ (10, 10)B-N-NTs from inner to outer tubes is found to be 0.53, 0.89, and 0.71 and 0.15electrons respectively, which is an acceptable value (Table1) and it is small for the (5, 5) @ (10, 10) structures due to their unstable forms (Fig 3).
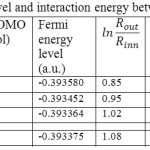 |
Table 1: Gap energy, Fermi level and interaction energy between two layers of DWBNNTs Click here to View table |
It is indicated in this study that the DWBNNTs can be used as a capacitor due to the semiconductor character, metallic behavior and dielectric function of SWBNNTs. Surprisingly, the optical properties93 of the SWBNNTs optimized by Guo94 found that the dielectric function93 could also be divided into two spectral regions94, namely, the high and the low energy region -energies these systems94.
We show that for the (5, 5) @ (8, 8) the distribution of the accumulated charges in the inner tubes are quantum-mechanically spilled outwards clearly. The capacitance (and) and dielectric constant of the various capacitors have been calculated based eq.4 and are listed in table. 2.
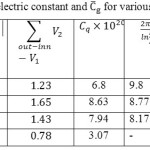 |
Table 2: The dielectric constant and Cg for various DWBNNTs
|
Conclusion
This work has been investigated the electric polarization and capacitance in a nano-scale coaxial-cylindrical-capacitors which is made of DW boron nitride, (n, n) @ (m, m), using ONIOM model including density-functional and semi empirical methods with the enforced Fermi-energies difference scheme. Despite the fact that the SWBNNTs@ SWBNNTs are a cylindrical capacitor, it has a very sensitive electrical storage compared to SWCNTs@ SWCNTs.
References
- M. Topsakal, S. Ciraci, Phys. Rev. B, 2012, 85, 045121. 18-20
- Klein, D. L.; Roth, R.; Lim, A. K. L.; Alivisatos, A. P.; McEuen, P. L. Nature (London) 1997, 389, 699
CrossRef - Ryo Nakanishi , , Ryo Kitaura , , Jamie H. Warner, Yuta Yamamoto ,, Shigeo Arai, Yasumitsu Miyata, & Hisanori Shinohara , SCIENTIFIC REPORTS | 3 : 1385 2013, DOI 10.1038 /srep01385
- Dai, H. J.; Rinzler, A. G.; Nikolaev, P.; Thess, A.; Colbert, D. T.; Smalley, R. E. Chem. Phys. Lett. , 1996, 260, 471
CrossRef - Sinnott, S. B.; Andrews, R.; Qian, D.; Rao, A. M.; Mao, Z.; Dickey, E. C.; Derbyshire, F. Chem. Phys. Lett. 1999, 315, 25,
CrossRef - Cheung, C. Li.; Kurtz,A.; Park,H.; Lieber,C.M.; J. Phys. Chem. B,2002, 106, 2429 2433
CrossRef - Z. M. Li, Z. K. Tang, H. J. Liu, N. Wang, C. T. Chan, R. Saito, S. Okada, G. D. Li J. S. Chen, N. Nagasawa, and S. Tsuda, Phys. Rev. Lett. 2001, 87, 127401
CrossRef - Z. K. Tang, L. Zhang, N. Wang, X. X. Zhang, G. H. Wen, G. D. Li, J. N. Wang, C. T. Chan, and P. Sheng, Science, 2001, 292, 2462,
CrossRef - D. Connétable, G. M. Rignanese, J. C. Charlier, and X. Blase, Phys. Rev. Lett. 2005, 94, 015503
CrossRef - P. M. Ajayan and S. Iijima, Nature, 1992, 358, 23
CrossRef - L. F. Sun, S. S. Xie, W. Liu, W. Y. Zhou, and Z. Q. Liu, Nature, 2000, 403, 384
CrossRef - N. Wang, Z. K. Tang, G. D. Li, and J. S. Chen, Nature 408, 50 2000. L. C. Qin, X. Zhao, K. Hirahara, Y. Miyamoto, Y. Ando, and S. Iijima, Nature 2000, 408, 50
CrossRef - X. Zhao, Y. Liu, S. Inoue, T. R. Suzuki, O. Jones, and Y. Ando, Phys. Rev. Lett. 2004, 92, 125502
CrossRef - T. Hayashi, Y. A. Kim, T. Matoba, M. Esaka, K. Nishimura, T. Tsukada, M. Endo, and M. S. Dresselhaus, Nano Lett. 2003 3, 887
CrossRef - A. Rubio, J. L. Corkill, and M. L. Cohen, Phys. Rev. 1994, B 49, 5801
- N. G. Chopra, R. J. Luyken, and K. Cherrey, Science, 1995, 269, 966
CrossRef - M. Monajjemi J Mol Model. 20(11):2507. doi: 10.1007/s00894-014-2507-y. Epub Oct 31, 2014
CrossRef - S. Okada, S. Saito, and A. Oshiyama, Phys. Rev. 2002, B 65, 165410
- Xiang, H., Yang, J., Hou, J. & Zhu, Q. Phys. Rev.2003, B 68, 035427
- Chen, Y., Zou, J., Campbell, S. J. & Le Caer, G. Appl. Phys. Lett.2004, 84, 2430–2432,
CrossRef - Kitaura, R. et al. Angew. Chem. Int. Ed. 2009, 48, 8298–8302
CrossRef - Zhao, X.,Ando, Y., Liu, Y., Jinno,M.&Suzuki, T. Phys. Rev. Lett.2003, 90, 187401
CrossRef - Shiozawa, H. Adv. Mater. 2008, 20, 1443–1449
CrossRef - Ando, Y. Chem. Phys. Lett. 2000, 323, 580–585
CrossRef - U. D. Venkateswaran Phys. Rev. B, 2002, 65, 054102
CrossRef - Gugang Chen,S. Bandow,E. R. Ma rgine,C. Nisoli,A. N. Kolmogorov,Vincent H. Crespi,R. Gupta,G.U. Sumanasekera,S. Iijima,and P. C. Eklund , PHYSICAL REVIEW LETTERS, 2003, 90, 25 , 257403, 1-4
- A. Loiseau , F. Willaime , N. Demoncy, G. Hug and H. Pascard , Phys. Rev. Lett. 1996, 76, 4737
CrossRef - M. Radosavljevic, J. Appenzeller, V. Derycke , R. Martel, Ph. Avouris, A. Loiseau, J.-L. Cochon and D. Pigache, Appl. Phys. Lett.2003, 82 ,4131
CrossRef - Y. Miyamoto, A. Rubio, M. L. Cohen and S. G. Louie, Phys. Rev. B, 1994, 50 ,4976
CrossRef - Y. H. Kim, K. J. Chang and S. G. Louie, Phys. Rev. B, 2001, 63, 205408
CrossRef - K. H. Khoo, M. S. C. Mazzoni and S. G. Louie, Phys. Rev. B 2004,69, 201401R
CrossRef - C. W. Chen, M. H. Lee and S. J. Clark, Nanotechnology, 2004, 15, 1837
CrossRef - M. Ishigami , J. D. Sau , S. Aloni, M. L. Cohen and A. Zettl , Phys. Rev. Lett.2005, 94 ,56804
CrossRef - Y. Miyamoto, A. Rubio , M.L. Cohen and S. G. Louie, Phys. Rev. B, 1994, 50 , 4976
CrossRef - E. J. Mele and P. Kral, Phys. Rev. Lett.2002, 88 , 56803
CrossRef - S. M. Nakhmanson, A. Calzolari,V. Meunier, J. Bernholc and M. B. Nardelli Phys. Rev. B,2003, 67, 235406
CrossRef - X. Bai, D. Golberg, Y. Bando, C. Zhi, C. Tang, M. Mitome and K. Kurashima ,Nano Lett.1997, 7, 632
CrossRef - Kazuyuki Uchida, Susumu Okada, Kenji Shiraishi and Atsushi Oshiyama J. Phys.: Condens. Matter,2007, 19 365218
CrossRef - Phys. Rev. B 76, 155436 – Published 30 October Kazuyuki Uchida, Susumu Okada, Kenji Shiraishi, and Atsushi Oshiyama, 2007
- Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013, 117, 1670 −1684
CrossRef - Mollaamin, F.; Monajjemi, M, Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
CrossRef - Mollaamin, F.; Varmaghani, Z.; Physics and Chemistry of Liquids. 2011, 49 318
CrossRef - Monajjemi, M. Chemical Physics. 2013,425, 29-45,
CrossRef - Monajjemi, M.; Wayne Jr, Robert. Boggs, J.E. Chemical Physics. 2014,433, 1-11
- Monajjemi, M.; Mollaamin, F. Journal of Computational and Theoretical Nanoscience, 2012, 9 (12) 2208-2214
CrossRef - Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics,2013, 19, 155–164
CrossRef - Monajjemi, M.; Mollaamin, F. Journal of Cluster Science, 2012, 23(2), 259-272
CrossRef - Tahan, A.; Monajjemi, M. Acta Biotheor,2011, 59, 291–312, (2011)
CrossRef - Honaparvar , B.; Khalili Hadad ,B.; Ilkhani ,AR.; Mollaamin, F. African Journal of Pharmacy and Pharmacology .2010, 4(8), 521 -529
- Mollaamin, F.; Monajjemi, M. Physics and Chemistry of Liquids , 2012, 50, 5, 2012, 596–604
- Monajjemi, M.; Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011,111, 2771–2777
CrossRef - Monajjemi, M. Theor Chem Acc, 2015,134:77 DOI 10.1007/s00214-015-1668-9
CrossRef - Jalilian, H.; Monajjemi, M. Japanese Journal of Applied Physics. 2015,54, 085101
CrossRef - Monajjemi, M.; Khaleghian, M.; Mollaamin, F. Molecular Simulation. 2010, 36, 11, 865–870
CrossRef - Soofi, N.S., Monajjemi, M, Oriental Journal of Chemistry. 2016, 32, 11, 2327-2345
- Elsagh, A., Zare, K., Monajjemi, M. Oriental Journal of Chemistry, 2016, 32, 5, 2585-2598
- Raoufi, F.; Aghaee, H.; Monajjemi, M. Oriental Journal of Chemistry, 2016,32, 4, 1839-1858
- Rahimi, A.;, Monajjemi, M, Oriental Journal of Chemistry, 2016, 32, 6, 2957-296559. Monajjemi, M. Struct Chem. 2012, 23,551–580
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
CrossRef - Mollaamin, F.; Gharibe, S.; Monajjemi, M. Int. J. Phy. Sci, 2011, 6, 1496-1500
- S. Iijima and T. Ichihashi, Nature, 1993, 363, 603
CrossRef - D.S. Bethune, e.B. Kiang, M.S. de Vries, G. Gorman, R Savoy, J. Vazquez, R Beyers, Nature 1993, 363, 605
CrossRef - J.W. Mintmire, B.I. Dunlap, and C.T. White, Phys. Rev. Lett. 1992, 68, 631
CrossRef - R Saito, M. Fujita, G. Dresselhaus, and M.S. Dresselhaus, Appl. Phys. Lett.1992, 60, 2204
CrossRef - G. Bilalbegovic, Phys. Rev. B,1998, 58, 15412
CrossRef - G. Bilalbegovic, Solid State Commun. (2000)
- G. Bilalbegovic, Computational Materials Science,2000, 18, 333-338
CrossRef - I. S. Gradshteyn and I. M. Ryzhik, Table of Integrals, Series, and Products (Academic Press, New York, (1965)
- W. A. de Heer, Rev. Mod. Phys.,1993, 65, 611
CrossRef - S. Wang and M. Grifoni, Phys.Rev.B, 2008, 77, 085431
CrossRef - M.W.Schmidt, K.K.Baldridge, J.A.Boatz, S.T.Elbert, M.S.Gordon, J.H.Jensen, S.Koseki, N.Matsunaga, K.A.Nguyen 2004, 14(11) 1347–1363
- Yan Zhao, Donald G. Truhlar, Theor Chem Account, 2008, 120 ,215–241
CrossRef - W. Kohn, L. J. Sham, Phys. Rev. 140 A ,1965, 1133-1138
- J.P. Perdew, K.Burke , Ernzerhof, Phys. Rev. Lett.1996, 77, 3865-3868
CrossRef - D. L. Klein, R. Roth, A. K. L. Lim, A. P. Alivisatos, and P. L. McEuen, Nature (London) 1997, 389 699
CrossRef - Yan Zhao, Donald G. Truhlar, Accounts of Chemical Research, 2008, 41(2) 157-167
CrossRef - C.E.Check, T.M.Gilbert, J. Org. Chem, 2005, 70 ,9828–9834
CrossRef - S.Grimme, Seemingly. Angew. Chem., Int. Ed.2006, 45 ,4460–4464
CrossRef - B.H. Besler, K.M. Merz, P.A. Kollman, J. comp. Chem.1990, 11 ,431
- L.E. Chirlian , M.M. Francl, J.comp.chem.1987, 8 ,894
- Brneman GM, Wiberg KB ,J. Comp Chem, 1990, 11: 361
CrossRef - Martin F, Zipse H . J Comp Chem. 2005, 26: 97 ,105
- Monajjemi M, Lee VS, Khaleghian M, Honarparvar B, Mollaamin F ,J. Phys. Chem. C, 2010, 114 15315
CrossRef - M. Monajjemi M , Khaleghian M J Clust Sci, 2011, 22:673–692
CrossRef - Monajjemi M Struct. Chem.2012, 23: 551
CrossRef - Balderchi, A, Baroni S, Resta R Phys. Rev. Lett 1998, 61: 173
- Tian Lu, Feiwu Chen, Acta Chim. Sinica, 2011, 69, 2393-2406
- Tian Lu, Feiwu Chen, J. Mol. Graph. Model. 2012, 38, 314-323
CrossRef - Tian Lu, Feiwu Chen, Multiwfn: J. Comp. Chem.2012, 33, 580-592
CrossRef - X. Blase, Phys. Rev. Lett.1994, 72, 1878
CrossRef - E. Hernández, C. Goze, P. Bernier, and A. Rubio, Phys. Rev. Lett.1998, 80, 4502
CrossRef - L. Wirtz, A. Rubio, R. A. Concha, and A. Loiseau, Phys. Rev. B, 2003, 68, 045425
CrossRef - G.Y. Guo, J.C. Lin, Phys. Rev. B, 2005, 71, 165402
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









