Synthesis of Gold Nanoparticles using Schiff Base Derivative of Ceftriaxone Sodium With Isatin as A Reducing and Stabilizing Agent
Ahlam Jameel Abdulghani and Saja Khalil Mohuee
University of Baghdad, Department of Chemistry, College of Science, Jaderiya, Baghdad, Iraq.
Corresponding author E-mail: almumaraj@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330327
Gold nanoparticles AuNPs were synthesized in aqueous solutions at different conditions via the reduction of sodium tetrachloroaurate (III) (NaAuCl4) by the Schiff base ligand sodium (6S)-7-((Z)-2-(methoxyimino)-2-(2-((Z)-2-oxoindolin-3-ylideneamino)thiazol-4-yl)acetamido)-3-((2-methyl-6-oxido-5-oxo-2,5-dihydro-1,2,4-triazin-3-ylthio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate (ISCR) derived from the condensation reaction of ceftriaxone sodium (CR) with 1H-indole-2,3-dione (isatin, Is). The synthesized AuNPs were characterized by UV- visible spectroscopy, FTIR spectroscopy, X-ray diffraction (XRD), scanning electron microscope (SEM), and atomic force microscope (AFM).
KEYWORDS:Ceftriaxone; Isatin; Schiff base; AuNPs; surface plasmon bands
Download this article as:| Copy the following to cite this article: Abdulghani A. J, Mohuee S. K. Synthesis of Gold Nanoparticles using Schiff Base Derivative of Ceftriaxone Sodium With Isatin as A Reducing and Stabilizing Agent. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Abdulghani A. J, Mohuee S. K. Synthesis of Gold Nanoparticles using Schiff Base Derivative of Ceftriaxone Sodium With Isatin as A Reducing and Stabilizing Agent. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33816 |
Introduction
The synthesis of AuNPs via the reduction of AuCl4– in aqueous solution by using the antibiotic ceftriaxone(CR) has been reported earlier [1]. 1H-indole-2,3-dione (isatin, Is) and its Schiff base with β- lactam antibiotic cefotaxime were also reported as reducing and stabilizing agents in the synthesis of AuNPs [2, 3]. The sizes, shapes and stability of the prepared AuNPs were found to change with type of ligand, reactant concentrations, pH and reaction temperature. The condensation of isatin with cefotaxime was found to decrease the rate of reduction of Au(III) ions compared with the free isatin molecules [2, 3] . In this work the reduction of AuCl4– to AuNp in aqueous solution is investigated at different conditions , using the Schiff base ligand sodium (6S)-7-((Z)-2-(methoxyimino)-2-(2-((Z)-2-oxoindolin-3-ylideneamino)thiazol-4-yl)acetamido)-3-((2-methyl-6-oxido-5-oxo-2,5-dihydro-1,2,4-triazin-3-ylthio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate (ISCR) ( Fig. 1) that has been previously prepared from the condensation reaction of ceftriaxone antibiotic(CR) with isatin [4]
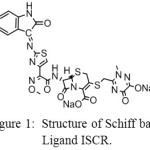 |
Figure 1: Structure of Schiff base Ligand ISCR. Click here to View figure |
Materials and Methods
Materials
Ceftriaxone sodium (C18H16N8O7S3Na2.3.5H2O) (LDP), isatin (Aldrich), sodium tetrachloroaurate (III) dihydrate (NaAuCl4.2H2O) (BDH), were used as received from suppliers. Buffer solutions of different pH were prepared from potassium dihydrogen orthophosphate KH2PO4 99% (Fluka), dipotassium hydrogen orthophosphate, K2HPO4, 99%, (Fluka) and phosphoric acid H3PO4 (Analar,BDH). The synthesis and characterization of the the Schiff base ligand ISCR has been reported earlier([4]
Instumentation
Electronic spectra of the prepared AuNPs solutions were obtained on a (200–1100 nm) SHIMADZU 1800 Double Beam UV-Visible spectrophotometer. The binding of Schiff base ligand to AuNPs was analyzed by FTIR spectra using a SHIMADZU FT-IR 8400S spectrophotometer. Size and morphology of AuNPs were determined by Scanning electron microscope (SEM) and by atomic force microscopy AFM images using SEM (KYKY-EM3200) and AFM model AA 3000 SPM 220 V-Angstrom (Advanced INC. USA). XRD analyses were measured by using a SHIMADZU XRD-6000 x-ray diffract ometer with a Cu-Kα (λ = 0.154060 nm) radiation source.
Synthesis of Aunps at Different Conditions
An aqueous solution of the Schiff base ligand ISCR (Na2C26H19N9O8S3.2H2O. 1.5CH3OH ) (1.266×10-4 M) was prepared by dissolving 0. 1027 g of the ligand in 1000 ml deionized water (DDW). A stock aqueous solution of AuCl 4– ( 2.514×10-3 M) was prepared by dissolving 0.1 g of NaAuCl4.2H2O in 100 ml distilled deionized water (DDW) in 100 ml volumetric flask. A standard solution of AuCl 4–(2.514×10-4 M) was prepared by diluting 10 ml of the stock solution to 100 ml. Ten aqueous solutions containing a constant concentration of NaAuCl4.2H2O solution (7.542×10-5 M) and different concentrations of ISCR (6.33×10-6, 1.266×10-5, 1.899×10-5, 2.532×10-5, 3.165×10-5, 3.798×10-5, 5.064×10-5, 6.33×10-5, 7.596×10-5 and 8.862×10-5 M) were prepared by adding 0.25, 0.5, 0.75, 1.0, 1.25,1.5, 2, 2.5, 3, 3.5 ml of a freshly prepared solution of ISCR (1.266×10-4 M) to 1.5 ml of AuCl 4– solution (2.514×10-4 M) followed by dilution to 5ml. The concentration ratios of ISCR / AuCl4– = 0.084, 0.168, 0.252, 0.336, 0.42, 0.504, 0.671, 0.84, 1.007 and 1.175 respectively. The uv-visible spectra of the prepared solutions were measured at room temperature to obtain the optimum concentration ratio of ISCR/ AuCl 4– . The effect of temperature on the synthesis rate of AuNPs was studied spectrophotometrically by heating ten (5 ml) solutions of the selected concentration ratio of ISCR/ AuCl4–, for 5 minutes at 35, 40, 45, 50, 55, 60, 65, 70, 75 and 80 ºC respectively and at selected temperature for different heating times (5, 10, 15, 20, 25, 30, 35, 40, 45 and 60 min) in a water bath. The pH effect on AuNPs synthesis was also studied spectrophtometrically, using the selected ISCR/ AuCl4– ratio at pH media : 2.37, 3.25, 4.15, 5.70, 6.30, 7.22, 8.38, 8.83, 10.14 and 11.16.
Results and Discussion
Uv-Vis Spectrophotometry
Concentration Effect
Fig. 2 shows the uv-visible spectra of Schiff base ligand, ISCR(a), AuCl4– (b) and AuNPs solution prepared from mixing 1.5 mL of ISCR (1.266×10-4 M) with 0.75 mL of AuCl4– (2.514×10-4 M) diluted to 5 mL(c). The spectrum of ISCR displayed two bands at λ 230 and 297 nm attributed to π→π* transition as was reported earlier[4], while the spectrum of AuCl4– solution corresponds to square planar tetrachoroaurate complex [5,6]. After 24h of preparation, the solution mixture developed a pink color with absorption maxima at 533 nm assigned to the surface plasmon band SPB of spherical AuNPs[7-13]. This indicates that AuCl4– has been reduced by Schiff base ligand to form AuNPs.
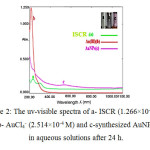 |
Figure 2: The uv-visible spectra of a- ISCR (1.266×10-4 M), b- AuCl4– (2.514×10-4 M) and c-synthesized AuNPs in aqueous solutions after 24 h. Click here to View figure |
Fig. 3 shows the variation in the absorption spectra of AuNPs solutions prepared from a constant concentration of AuCl4– (3.771×10-5 M) and different concentrations of ISCR (6.33×10-6, 1.266×10-5, 1.899×10-5, 2.532×10-5, 3.165×10-5, 3.798×10-5, 5.064×10-5, 6.33×10-5, 7.596×10-5 and 8.862×10-5 M). (1-10 respectively). No color change and no SPB were detected in all solutions until 24 h when the spectra gave increased absorption of single bands at λ=(538, 538, 534, 549, 541, 546, 541, 541, 538 and 535 nm respectively assigned to SPR of spherical AuNPs [ 7-12]. After 48h the spectra of all solutions showed increased absorptions with the bands of solutions 1, 4, 6-10 being shifted to lower wavelengths and appeared at λ= (531, 542, 541, 538, 535, 537 and 533 nm respectively). Absorption bands of solutions 2, 3 and 5 were shifted to higher wavelengths and appeared at λ= 541, 545, 543 nm respectively, referring to larger size or aggregation of AuNPs. After one week, solution 2 exhibited two high absorption bands appeared at 538 and 776 nm which may be attributed to the formation of non-spherical AuNPs[5, 14-17] . After three weeks, band absorptions increased and their positions were shifted to higher wavelengths and appeared at λ= 560, (540 and 830), 540, 542, 540, 538, 543, 543, 540 and 542 nm respectively. After four weeks the intensity of SPBs decreased. The best performance was recorded by solutions 9 and 10 (ISCR/AuCl4– 1.007 and 1.175 respectively) the spectra of which exhibited higher SPB absorbance and higher stability with time compared with the other solutions. This result is quite different from those reported on using ceftriaxone (CR) and isatin (Is) separately when optimum concentration ratios of CR/ AuCl4– and Is/ AuCl4– were (0.172) [1] and (9.52) [2] respectively which reflects the effect of different ligand structures on gold nanoparticle synthesis.
 |
Figure 3: Absorption spectra of ISCR- synthesized AuNPs at different concentration ratios of ISCR / AuCl4– (0.084, 0.168, 0.252, 0.336, 0.42, 0.504, 0.671, 0.84, 1.007 and 1.175 (1-10 respectively). Click here to View figure |
Temperature Effect
Fig. 4 shows the spectra of ten solutions of ISCR / AuCl4– (1.007) after being heated at 35-80°C for 5 minutes. All solutions exhibited weak absorption bands appeared at λ = 549, 550,
 |
Figure 4: Absorption spectra with time for AuNPs synthesized from a solution of ISCR / AuCl4– 1.007 heated at 35° -80°C. Click here to View figure |
540,536, 539, 535, 529, 534, 531 and 525 nm respectively, assigned to the SPB of spherical AuNPs with estimated size range about 10-60 nm [7, 9,10,13]. The reaction time to form AuNPs was reduced with increasing temperatures and all solutions exhibited continuous increase in absorbance of SPBs for more than 48h. As in the case of CR[1], the highest rate of GNPs synthesis was recorded at 80 °C which remained stable for more than two weeks . Fig. 5 shows the spectra of AuNPs in ten solutions of the concentration ratio ISCR / AuCl4– (1.007) heated at 80°C for 5 min to 60 min. The spectra of all solutions exhibited single absorption bands in the visible region appeared at λ= (524, 529, 538, 530, 528, 541, 534,
 |
Figure 5: Absorption spectra of AuNPs prepared from a solution of ISCR/ AuCl4– (1.007) heated at 80°C for different time (5-60 min). Click here to View figure |
534, 530 and 533 nm respectively. After 24h the bands of solutions heated at 5, 25, 40, 45 and 60 min. were shifted to higher wavelengths and appeared at (530, 531, 538, 533 and 534 nm respectively), while those of the solutions heated for 15,30 and 35 min were shifted to lower wavelength and appeared at λ=(531, 537 and 519 nm respectively). The spectra of all solutions showed increased absorption of SPB with time for one week, followed by decreased absorption after two weeks. The best heating times were 5 and 30 minutes. However, the reduction process by ISCR was of lower rate compared with that of free CR and isatin[1,2] which may be attributed to the removal of free amino group of CR moiety and the change redox behavior of isatin moiety .
PH Effect
Fig. 6 shows the absorption spectra of ISCR- synthesized AuNPs solutions at different pH media (2.37, 3.25, 4.15, 5.70, 6.30, 7.22, 8.38, 8.83, 10.14 and 11.16, 1-10 respectively) using ISCR / AuCl4– concentration ratio (1.007).
 |
Figure 6: Absorption spectra of AuNPs synthesized at different pH value. Click here to View figure |
After 1h of preparation no color change and no SPB were detected in all solutions. After 24h only the solutions (2, 3 and 4) of pH (3.25-5.70) gave pink colors and their spectra exhibited a single weak absorption band at λ 533, 540 and 537 nm respectively. The best performance was exhibited at pH=5.7 and to less extent at pH= 4.15 as the two solutions remained stable for two weeks. Despite the difference in ligand / AuCl4– ratio, the performances of ISCR were almost similar to that of CR and Is at nearly the same pH values [1, 2]. The low rate of reduction of Au(III) in highly acidic solutions may be attributed to the protonation of functional groups responsible for electron donation while neutral and basic solutions may result in the formation of stable Au(III) hydroxyl anion complexes such as [AuCl3(OH)]–, [AuCl2(OH)2]–, [AuCl(OH)3]– or [Au(OH)4]– [18,19]. The reduction process by ISCR was also of lower rate compared with that of CR and isatin[1,2].
Characterization of AuNPs
FT-IR Spectra
The infrared band assignment of the FTIR spectrum of ISCR shown in Fig. 7 a was reported earlier [4]. The spectrum of ISCR- synthesized AuNPs (Fig. 7b) shows that the positions of the bands attributed to azomethine (-HC=N-)(1625.88 cm-1) , υ C=O of lactam(1733.89 cm-1), υ C=O overlapped amide and ester(1660.10 cm -1), νasy(COO–) and νsy(COO–) (1640.25 and 1430.15 cm-1 respectively) of the free ligand were shifted and appeared at 1604, 1680, 1641.31 and (1646 and 1434) cm-1 respectively. The two bands observed at 3425.34 and 3236.33 cm-1 in Fig. 7b may be assigned to N-H stretching vibration of NH2 group attached to the benzene ring of isatin moiety. This band may be resulted from the oxidation of the indole ring leading to ring opening [2]. These data refer to the binding of AuNPs with functional groups of the Schiff base ligand.
 |
Figure 7: FT-IR spectrum of a- ISCR and b- ISCR – synthesized AuNPs |
X-Ray Diffraction (XRD)
Fig. 8 shows the diffraction pattern of AuNPs prepared from aqueous solution of ISCR / AuCl4– = (1.007). Four diffraction peaks were observed at 2θ = (38.2522, 44.4910, 64.6450 and 77.919) degrees corresponding to the planes (111), (200), (220) and (311) of face centered cubic lattice structure of AuNPs [9, 20] which are in agreement with the XRD pattern data available in the JCPDS file no.(04-0784). The crystalline sizes of the nanoparticle were estimated using unmodified Scherrer’s equation [21, 22]. The average size was found 38.5 nm corresponding to the planes (111) and (200), and 80 nm according to the planes (111), (200), (220) and (311).
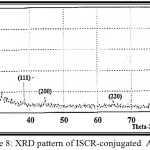 |
Figure 8: XRD pattern of ISCR-conjugated AuNPs Click here to View figure |
Scanning Electron Microscopy (SEM
The SEM image of AuNPs at room temperature and at heating temperature range 35-80°C for 5 min. showed particles of regular spherical shapes with a wide size distribution ranging between 44-97 nm and average size of 73 nm (Fig. 9). However, heating the aqueous solution at 80°C for 30 minutes gave rise to different shapes and sizes of AuNPs which appeared as spherical, nanorods and irregular shapes as is shown in Fig.10. The average diameter of nanosphers was 43.39 nm, while average diameter and length of nanorods were 43 and 178 nm respectively. This result shows that the heating period at 80°C affects the size and morphology of AuNPs in this study.
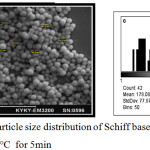 |
Figure 9a: SEM b-particle size distribution of Schiff base ligand -synthesized AuNPs heated at 80°C for 5min |
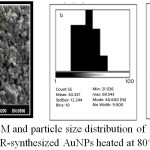 |
Figure 10: a -SEM and particle size distribution of spherical (b) and rod like(c) ISCR-synthesized AuNPs heated at 80°C for 30 min. Click here to View figure |
Atomic Force Microscopy (AFM)
Fig. 11 shows the AFM micrographs of the ISCR- synthesized AuNPs at heating temperature 80°C for 5 minutes which have spherical shapes with average diameter of 82.28 nm. The AFM image of the same solution heated at 80°C for 30 minutes( Fig.12) showed the presence of different sizes and shapes of AuNPs with average particle sizes 85.52 nm.
 |
Figure 11: AFM pictures and size distribution of ISCR-synthesized AuNPs heated at 80°C for 5 min . Average diameter 82.28 nm. Click here to View figure |
 |
Figure 12: AFM pictures and size distribution of ISCR-synthesized AuNPs heated at 80°C for 30 min. Average diameter 85.52 nm. |
Antibacterial Activity
The antibacterial activity of ISCR and ISCR-capped Au NPs have been tested against the pathogenic bacteria gram negative Escherichia coli, and gram positive Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pneumonia compared with the activity of CR and CR-capped AuNPs that has been reported earlier[1], using drop diffusion method. The diameter of inhibition zones caused by each test sample was measured in (mm) and the results are described in Table 1. The ISCR-capped AuNPs showed the highest activity against all bacterial cultures compared with CR, ISCR and CR- capped AuNPs. These results indicate that AuNPs enhanced the activity of Schiff base ligand especially against Pseudomonas Aeruginosa and Staphylococcus aurous. The Escherichia coli was more sensitive to the four tested solutions compared with the other three cultures.
Table 1: Inhibition zones (mm) exhibited by CR, ISCR and their conjugated AuNPs against some pathogenic bacteria.
|
|
Inhibition zone (mm) |
|||
|
Sample |
Escherichia Coli |
Pseudomonas Aeruginosa |
Staphylococcus aurous |
Streptococcus Pneumonia |
|
CR[1] |
17.5 |
– |
1 |
– |
|
CR-capped AuNPs[1] |
20 |
– |
1 |
1 |
|
ISCR |
20 |
– |
– |
3 |
|
ISCR-capped AuNPs |
25 |
7.5 |
10 |
5 |
Conclusions
AuNPs conjugates were successfully synthesized from the reduction of Au(III) ions with the Schiff base derivative of ceftriaxone antibiotic with isatin (ISCR) at different concentration ratio of ISCR /AuCl4– , temperature and pH media. The particles were characterized by uv-visible spectroscopy and XRD analysis, and their conjugation with ISCR functional groups was proved by FTIR spectroscopy. The optimum conditions for AuNPs synthesis were ISCR /AuCl4– 1.007, pH 5.7, at 80 °C. Heating at 80 °C for 30 minutes changed the morphology of synthesized AuNps from totally regular spherical shapes to a mixture of spherical, nanorods and irregular shapes. The condensation of amino group with the carbonyl group of isatin was found to decrease the rate of AuNPs synthesis compared with the performance of the two free separate molecules. Conjugation of ISCR with AuNPs enhanced its antibacterial activity.
References
- Abdulghani, A. J.; Mohuee, S. K. Iraqi Journal of Science. 2015, 56(3C), 2425-2438.
- Abdulghani A. J.; Hussain, R. K. Baghdad Science Journal. 2014, 11(3), 1201-1216)
- Abdulghani, A. J.; Hussain, R. K. Journal of Chemical, Biological and Physical Sciences, Section A, Chemical Sciences. 2015, 5(4), 3668-3684.
- Abdulghani, A. J.; Mohuee, S. K. Journal of Chemical, Biological and Physical Sciences, Section A. 2016, 6(2), 579-595.
- Link, S.; El-Sayed, M. A. The Journal of Physical Chemistry, 1999, 103, 4212-4217.
CrossRef - Jiang, G.; Wang, L.; Chen, W. Materials Letters, 2007, 61, 278–283.
CrossRef - Bhattacharya , D. ; Saha, B. ; Mukherjee, A. ; Santra, C.R.; Karmakar, P. Nanoscience and Nanotechnology, 2012, 2(2), 14-21.
CrossRef - Zhang, L.; Swift, J.; Butts, C. A. ; Yerubandi, V. ; Dmochowski, I. J. Journal of Inorganic Biochemistry, 2007, 101, 1719–1729.
CrossRef - Rai, A.; Prabhune, A.; Perry, C. C. Journal of Materials Chemistry, 2010,20, 6789–6798.
CrossRef - Demurtas, M.; Perry, C. C. Gold Bull, , 2013, 47(2014), 103–107.
CrossRef - Brown, A.; Smith, K.; Samuels, T. A.; Lu, J.; Obare, S.; Scott, M. E. Applied and Environmental Microbiology, 2012, 78(8), 2768 –2774.
CrossRef - Qian, L.; Sha, Y.; Yang, X. Thin Solid Films, 2006,515, 1349–1353.
CrossRef - Jayalakshmi, K.; Ibrahim, M.; Rao, K. V. International Journal of Electronic and Electrical Engineering, 2014, 7(2), 159-164.
- Boopathi, S.; Senthilkumar, S.; Phani, K. L. Journal of Analytical Methods in Chemistry, 2012,212, Article ID 348965, Doi:10.1155/2012/348965,6 pages.
CrossRef - Johan, M. R; Chong, L. C.; Hamizi, N. A. International Journal of Electrochemical Science, 2012, 7, 4567-4573.
- Singh, P. P.; Bhakat, C. Chemistry and Materials Research, 2012, 2(1), 82-87.
- Shi, W.; Casas, J.; Venkataramasubramani, M.; Tang, L. International Scholarly Research Network ISRN Nanomaterials, 2012, ID 659043, doi:10.5402/2012/659043, 9 pages.
CrossRef - Young, J. K.; Lewnski, N. L.; Langsner, R. J.; Kennedy, L. C.; Satyanarayan, A.; Nammalvar, V.; Lin, A. Y.; Drezek, R. A. Nanoscale Research Letters, 2011,6(421), 11pages.
- Majziki, A.; Fulop, L.; Scapo, E.; Bogar, F.; Martinek, T.; Penke, B.; Biro, G.; Dekany, I. Colloids Surf. B: Biointerfaces, 2010, 81, 235-241.
CrossRef - Zhang, Y.; Wei, S.; Chen, S., International Journal of electrochemical science, 2013, 8, 6493 – 6501.
- Monshi, A.; Foroughi, M. R.; Monshi, M. R. World Journal of Nano Science and Engineering, 2012, 2, 154-160.
CrossRef - Firdhouse, M. J.; Lalitha, P.; Sripathi, S. K. Digest Journal of Nanomaterials and Biostructures, 2014, 9(1), 385 – 39

This work is licensed under a Creative Commons Attribution 4.0 International License.









