Synthesis and Antimicrobial Activity of Some Novel Pyrazolones
S. D. Mhaske1, S. J. Takate2, R. N. Dhawale2, H. N. Akolkar1, P. V. Randhavane1 and B. K. Karale1
1Department of Chemistry, Radhabai Kale Mahila Mahavidyalaya, Ahmednagar, 414001, India.
2Department of Chemistry, New Arts, Commerce and Science College, Ahmednagar, 414001, India.
Corresponding Author E-mail: bkkarale@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330330
Knoevenagel condensation was carried out using heterocyclic aldehydes and pyrazolone derivatives. The structure elucidation of condensation products 1, 2, 3 and 4 was done using spectral methods like IR, 1H NMR and mass spectrometry. These novel compounds were screened for antimicrobial activity.
KEYWORDS:Pyrazolone; Knoevenagel condensation; Antimicrobial activity
Download this article as:| Copy the following to cite this article: Mhaske S. D, Takate S. J, Dhawale R. N, Akolkar H. N, Randhavane P. V, Karale B. K. Synthesis and Antimicrobial Activity of Some Novel Pyrazolones. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Mhaske S. D, Takate S. J, Dhawale R. N, Akolkar H. N, Randhavane P. V, Karale B. K. Synthesis and Antimicrobial Activity of Some Novel Pyrazolones. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33137 |
Introduction
Multi drug resistant micro-organisms and increased systemic as well as infectious diseases are the two major challenges for scientific world. Development of newer synthetic entities can offer a major solution for these problems.
Pyrazolone containing compounds are associated with antimicrobial1, antiviral2, antifungal3, antioxidant4, cytotoxic5, analgesic6, anti-inflammatory7activities.Thiophene derivatives have found very important place in the field of drug discovery because of their potential biological activities8.
Thiazoles have found applications in drug development for treatment of HIV infections9, hypertension10 and as inhibitors of bacterial gyrase B11. Moreover pyrazole containing compounds are reported to have good biological activities like antimicrobial12, antifungal13, antiviral14, analgessic15, antiparastic16 and antineoplastic17.
Pyridine nucleus is important nitrogen heterocycle which exhibits antimicrobial18, anticancer19 and antioxidant20 activities. Imidazole derivatives have been shown to exhibit antibacterial 21, antifungal and antioxidant22 activity.
It has been shown that substitution pattern of bromine can alter the activity of a compound23. Fluorine containing compounds are associated with bioactivities such as antibacterial24, anticancer25 and analgesic26.
Knoevenagel condensation 27 reactions have occupied an important place in synthetic organic chemistry due to its efficiency in carbon-carbon bond formation. Many researchers have studied Knoevenagel condensation reactions of various aldehydes with pyrazolones28-29.
Activities associated with pyrazolones, thiophenes, pyrazoles, imidazoles and halogens prompted us to synthesize compound containing these moieties.
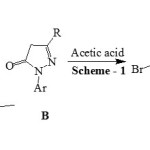 |
Scheme 1 |
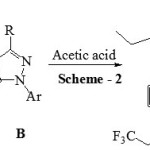 |
Scheme 2 |
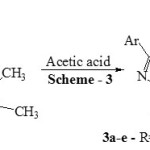 |
Scheme 3 |
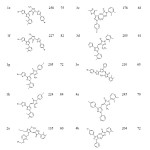 |
Table 1: Physical data of synthesized compounds |
Experimental
Physical constants were determined in open capillary. IR of the compounds were recorded using IRAffinity-1 Fourier transform infrared spectrophotometer (Shimadzu). PMR spectra were taken using Bruker Avance II 400 MHz NMR spectrometer. TMS was used as an internal standard and DMSO-d6 as a solvent. The mass spectra were recorded on HP 1100 LC/MS.
(4E)-4-((3-(5-Bromothiophen-2-Yl)-1-(2,3-Dimethylphenyl)-1H-Pyrazol-4-Yl)Methylene)-1-(2,3-Dimethylphenyl)-3-Propyl-1H-Pyrazol-5(4H)-One, 1
The mixture of 3-(5-bromothiophen-2-yl)-1-(2,3-dimethylphenyl)-1H-pyrazole-4-carbaldehyde A (0.01 mol) and 2-(2,3-dimethylphenyl)-5-propyl-2,4-dihydro-3H-pyrazol-3-one B (0.01mol) in 5 mL of glacial acetic acid was refluxed in round bottom flask for 30 min. Reaction progress was studied and confirmed using TLC at the end of reaction, reaction mixture was poured over ice. Product obtained 1 was separated using simple filtration then purified by recrystallization from ethanol. Compounds 2, 3 and 4 were synthesized using same procedure. Physical data of all the synthesized compounds is given in Table 1 and spectral data as below.
MS: m/z 572 (M+); PMR: δ 9.74 (s, 1H),7.70 (s, 1H),7.37 (d,1H, J = 3.8 Hz),7.32 (d, 2H, J = 3.8 Hz), 7.25-7.26 (m, 2H), 7.11-7.17 (m, 3H), 2.68 (t, 2H),2.36 (s, 3H), 2.31 (s, 3H), 2.12 (s, 3H), 2.08 (s, 3H), 1.77 (sextet, 2H), 1.04 (t, 3H); IR: 3132, 2958, 2918, 1676, 1554, 790, 754, 717, 663 cm-1.
MS: m/z 598 (M+); PMR: δ 9.76 (s, 1H), 7.81 (s, 1H), 7.42 (d,1H, J = 4 Hz), 7.34 (d, 1H, J = 4.8 Hz), 7.27-7.31 (m, 4H), 7.18 (t, 2H, J = 7 Hz), 2.35 (s, 3H), 2.32 (s, 3H), 2.11 (s, 3H), 2.06 (s, 3H); IR: 3122, 2920, 1691, 1593, 1112, 794, 754, 713, 619 cm-1.
MS: m/z 544 (M+); PMR: δ 9.71 (s, 1H),7.84-7.93 (m, 5H), 7.66 (s, 1H), 7.56-7.58 (m, 3H), 7.42 (d,1H, J = 3.9 Hz), 7.33 (d, 1H, J = 3.9 Hz), 2.80 (t, 2H), 2.33 (s, 3H), 2.15 (s, 3H), 1.81 (sextet, 2H), 1.08 (t, 3H); IR: 3128, 2952, 2911, 1669, 1544, 792, 758, 719 cm-1.
MS: m/z 570 (M+); PMR: δ 9.82 (s, 1H), 8.69 (s, 1H),7.87-8.09 (m, 5H), 7.51-7.54 (m, 3H), 7.42 (d, 1H, J = 3.8 Hz), 7.33 (d,1H, J = 3.8 Hz),2.38 (s, 3H), 2.12 (s, 3H); IR: 3122, 2925, 1693, 1597,1120, 790, 717, 688 cm-1.
MS: m/z 707 (M+); PMR: δ 9.76 (s, 1H), 7.93 (d, 2H, J =7.8 Hz), 7.70 (s, 1H), 7.36-7.41 (m, 4H), 7.31-7.33 (m, 3H),7.16 (t, 1H), 2.71 (t, 2H, J =7.4 Hz), 2.39 (s, 3H), 2.14 (s, 3H), 1.81 (sextet, 2H, J =7.4 Hz), 1.07 (t, 3H, J = 7.4 Hz); IR: 3128, 2926, 1693, 1597,1120, 790, 756, 715, 629 cm-1.
MS: m/z 731 (M+); PMR: δ 9.79 (s, 1H), 7.79 (s, 1H), 7.85 (d, 2H, J =7.7 Hz), 7.49 (d, 2H, J =7.7 Hz), 7.42 (d, 1H, J = 3.88 Hz),7.30-7.35 (m, 5H), 2.38 (s, 3H), 2.14 (s, 3H); IR : 3127, 2924, 1697, 1596, 1117, 793, 757, 718, 639 cm-1.
MS: m/z 562 (M+); PMR: δ9.73 (s, 1H), 7.93 (dd, 2H, J = 9.2 & 5 Hz),7.73 (s, 1H),7.44 (d, 1H, J = 3.8 Hz), 7.37-7.39 (m, 2H), 7.30-7.33 (m, 2H), 7.22 (t, 2H, J = 8.8 Hz), 2.72 (t, 2H), 2.38 (s, 3H), 2.12 (s, 3H), 1.78 (sextet, 2H, J = 7.3 Hz), 1.04 (t, 3H); IR : 3135, 2968, 2922, 1671, 1559, 788, 757, 722, 687 cm-1.
MS: m/z 588 (M+); PMR: δ 9.82 (s, 1H), 7.88 (dd, 2H, J = 9.12 & 4.92 Hz), 7.75 (s, 1H), 7.45 (d, 1H, J = 3.88 Hz), 7.36-7.39 (m, 2H), 7.32-7.35 (m, 2H), 7.22 (t, 2H, J = 8.8 Hz), 2.38 (s, 3H), 2.12 (s, 3H); IR: 3133, 2929, 1690, 1593,1123, 798, 755, 722, 689 cm-1.
MS: m/z 601 (M+); PMR: δ 8.18 (d,1H, J = 5.7 Hz), 7.48 (s, 1H), 7.45 (t, 1H, J = 8Hz), 7.09-7.18 (m, 2H), 7.02 (d, 1H, J = 5.7 Hz), 5.46 (s, 2H), 4.81 (q, 2H), 2.67 (t, 2H), 2.61 (t, 2H), 2.33 (s, 3H), 2.22 (t, 3H), 2.12 (t, 3H), 1.78 (sextet, 2H), 1.59 (quintet, 2H), 1.32 (sextet, 2H), 1.05 (t, 3H), 0.84 (t, 3H, J = 7.32 Hz); IR: 2928, 1656, 1623, 1558, 1234, 1126, 967, 769, 754, 711 cm-1.
MS: m/z 627 (M+); PMR: δ 8.16 (d,1H, J = 5.7 Hz), 7.50 (s, 1H), 7.43 (t, 1H, J = 8 Hz), 7.09-7.20 (m, 2H), 7.04 (d, 1H, J = 5.7 Hz), 5.45 (s, 2H), 4.80 (q, 2H), 2.62 (t, 2H), 2.32 (t, 3H), 2.21 (t, 3H), 2.14 (t, 3H), 1.57 (quintet, 2H), 1.33 (sextet, 2H), 0.85 (t, 3H, J = 7.4 Hz); IR: 2958, 2920, 1655, 1620, 1524, 1231, 1120, 967, 788 cm-1.
MS: m/z 734 (M+); PMR: δ 8.20 (d,1H, J = 5.7 Hz), 8.07 (s, 1H), 7.93 (d, 2H, J = 7.7 Hz), 7.50 (d, 2H, J = 5.8Hz), 7.47 (s, 1H), 7.05 (d, 1H, J = 5.7 Hz), 5.47 (s, 2H), 4.80 (q, 2H), 2.68 (t, 2H), 2.62 (t, 2H), 2.24 (s, 3H), 1.77 (sextet, 2H), 1.60 (quintet, 2H), 1.34 (sextet, 2H), 1.07 (t, 3H), 0.87 (t, 3H, J = 7.4 Hz); IR: 2927, 2918, 1676, 1622, 1525, 1231, 1123, 966 cm-1.
MS: m/z 760 (M+); PMR: δ 8.16 (d, 1H, J= 5.7 Hz), 8.06 (s, 1H), 7.91 (d, 2H, J = 7.8 Hz), 7.49 (d, 2H, J = 7.8 Hz), 7.48 (s, 1H), 7.05 (d, 1H, J= 5.7 Hz ), 5.48 (s, 2H), 4.81 (q, 2H), 2.63 (t, 2H), 2.22 (t, 3H), 1.62 (quintet, 2H), 1.32 (sextet, 2H), 0.88 (t, 3H, J = 7.3 Hz); IR: 2925, 2916, 1652, 1626, 1554, 790, 1233, 1126, 963, 755 cm-1.
MS: m/z 591 (M+); PMR: δ 8.20 (d, 1H, J = 5.5 Hz), 7.85-7.91 (m, 2H), 7.48 (s, 1H), 7.20 (t, 2H, J = 8.8 Hz), 7.03 (d, 1H, J = 5.5Hz), 5.47 (s, 2H), 4.78 (q, 2H), 2.66 (t, 2H), 2.62 (t, 2H), 2.21 (t, 3H), 1.76 (sextet, 2H), 1.57 (quintet, 2H), 1.33 (sextet, 2H), 1.05 (t, 3H), 0.84 (t, 3H, J = 7.4 Hz); IR: 2928, 2915, 1656, 1622, 1524, 1230, 1123, 966, 786 cm-1.
MS: m/z 617 (M+); PMR: δ 8.19 (d,1H, J = 5.5 Hz), 7.84-7.90 (m, 2H), 7.47 (s, 1H), 7.19 (t, 2H, J = 8.76 Hz), 7.01 (d, 1H, J = 5.6Hz), 5.47 (s, 2H), 4.83 (q, 2H), 2.63 (t, 2H), 2.21 (s, 3H), 1.60 (quintet, 2H), 1.32 (sextet, 2H), 0.85 (t, 3H, J = 7.34 Hz); IR: 2938, 2913, 1666, 1622, 1521, 1233, 1120, 966, 789 cm-1.
MS: m/z 563 (M+); PMR: δ 8.18 (d,1H, J = 5.6 Hz), 7.85-7.89 (m, 2H), 7.45 (s, 1H), 7.19 (t, 2H, J = 8.76 Hz), 7.03 (d, 1H, J = 5.7Hz), 5.49 (s, 2H), 4.81 (q, 2H, J = 8.52 Hz), 2.61 (t, 2H), 2.21 (s, 6H), 1.58 (quintet, 2H), 1.30 (sextet, 2H), 0.84 (t, 3H, J = 7.32 Hz); IR: 2922, 2918, 1651, 1622, 1523, 1230, 1122, 964, 781 cm-1.
MS: m/z 474 (M+); PMR: δ 10.21 (s, 1H), 7.89-7.92 (m, 2H), 7.83 (m, 1H), 7.57-7.61 (m, 4H), 7.42-7.47 (m, 3H), 7.32 (d, 1H, J = 7Hz), 7.21-7.24 (m, 2H), 2.68 (t, 2H), 2.45 (s, 3H), 2.34 (s, 3H), 2.08 (s, 3H), 1.77 (q, 2H), 1.04 (t, 3H); IR: 3136, 2972, 2929, 2872, 1678, 1598, 1508, 1500, 763 cm-1.
MS: m/z 494 (M+); PMR: δ 9.78 (s, 1H), 7.91 (d, 2H, J = 7.5 Hz), 7.70-7.75 (m, 2H), 7.60-7.63 (m, 2H), 7.58 (t, 2H, J = 8 Hz), 7.56 (s, 1H), 7.46 (t, 1H, J =7.5 Hz), 7.30 (d, 1H, J = 7 Hz), 7.18-7.24 (m, 2H),2.72 (t, 2H), 2.35 (s, 3H), 2.09 (s, 3H), 1.72 (sextet, 2H), 1.09 (t, 3H); IR: 3125, 2926, 1697, 1588, 1508, 1503, 1165, 835, 748 cm-1.
MS: m/z 515 (M+); PMR: δ 9.43 (s, 1H), 7.89-7.95 (m, 2H), 7.50 (s, 1H), 7.27-7.43 (m, 5H), 2.72 (s, 3H), 2.70 (t, 2H), 2.43 (s, 3H), 2.35 (s, 3H), 2.06 (s, 3H),1.76 (sextet, 2H), 1.02 (t, 3H); IR: 3120, 2922, 1695, 1598, 1508, 1500, 1155,833, 738 cm-1.
MS: m/z 494 (M+); PMR: δ 9.74 (s, 1H), 7.78 (s, 1H), 7.72 (dd, 1H, J =1.0 & 5.1 Hz),7.57 (dd, 1H, J =1.0 & 3.6 Hz), 7.34 (dd, 1H, J = 1.4 & 7Hz), 7.23-7.30 (m, 3H), 7.08-7.19 (m, 3H), 2.66 (t, 2H, J = 7.4 Hz), 2.34 (s, 3H), 2.29 (s, 3H), 2.11 (s, 3H), 2.06 (s, 3H), 1.75 (sextet, 2H, J = 7.4 Hz), 1.01 (t, 3H, J =7.4 Hz); IR: 3128, 2958, 1681, 1610, 1502, 786, 717 cm-1.
MS: m/z 621 (M+); PMR: δ 10.41 (s, 1H), 8.14 (s, 1H), 7.94 (d, 2H, J = 8.5 Hz), 7.78 (s, 1H), 7.68-7.70 (m, 2H), 7.63-7.66 (m, 5H), 7.36 (d, 1H, J = 7Hz), 7.21-7.27 (m, 2H), 2.66 (t, 2H, J = 7.4 Hz), 2.38 (s, 3H), 2.11 (s, 3H), 1.75 (sextet, 2H, J = 7.4 Hz), 1.01 (t, 3H, J = 7.4 Hz); IR: 3132, 2925, 1678, 1600, 1596, 1130, 814, 758 cm-1.
MS: m/z 500 (M+); PMR: δ 10.23 (s, 1H), 7.90-7.92 (m, 2H), 7.82 (s, 1H), 7.56-7.60 (m, 4H),7.41-7.47 (m, 3H),7.30 (d, 1H, J = 7 Hz), 7.19-7.26 (m, 2H), 2.45 (s, 3H), 2.35 (s, 3H),2.09 (s, 3H); IR : 3134, 2920, 1687, 1598, 1498, 1122, 839 cm-1.
MS: m/z 520 (M+); PMR: δ10.22 (s, 1H), 7.91 (d, 2H, J =7.5 Hz), 7.75 (s, 1H), 7.71-7.74 (m, 2H),7.65-7.67 (m, 3H),7.58 (t, 2H, J = 8 Hz), 7.45 (t, 1H, J = 7.5 Hz),7.18-7.25 (m, 2H),2.34 (s, 3H),2.08 (s, 3H); IR: 3120, 2922, 1687, 1602, 1496, 1124, 840, 756 cm-1.
MS: m/z 541 (M+); PMR: δ 9.46 (s, 1H),7.89-7.95 (m, 2H),7.50 (s, 1H), 7.26-7.45 (m, 5H), 2.71 (s, 3H), 2.43 (s, 3H), 2.36 (s, 3H), 2.08 (s, 3H); IR: 3120, 2916, 2848, 1693, 1600, 1155, 1124, 833, 738 cm-1.
MS: m/z 520 (M+); PMR: δ 9.78 (s, 1H), 7.81 (s, 1H), 7.78-7.79 (m, 1H), 7.48-7.49 (m, 1H), 7.26-7.37 (m, 5H), 7.15-7.22 (m, 2H), 2.35 (s, 3H), 2.31 (s, 3H), 2.12 (s, 3H), 2.06 (s, 3H); IR: 3107, 2951, 2920, 1685, 1598, 1120, 819, 786, 717 cm-1.
MS: m/z 647 (M+); PMR: δ 10.42 (s, 1H), 8.12 (s, 1H), 7.92 (d, 2H, J = 8.5 Hz), 7.76 (s, 1H), 7.69-7.70 (m, 2H), 7.62-7.64 (m, 5H), 7.33 (d, 1H, J = 7 Hz), 7.23-7.25 (m, 2H), 2.36 (s, 3H), 2.09 (s, 3H); IR: 3132, 2925, 1678, 1600, 1596, 1130, 814, 758 cm-1.
Results and Discussion
Pyrazolone derivatives were condensed with various aldehydes to obtain the products 1, 2, 3 and 4. Compound 1a in its IR spectrum shows carbonyl frequency band at 1676 cm-1 of pyrazolone ring. PMR spectrum of the compound exhibited signals of propyl side chain, a triplet at δ 1.04 due to methyl group, sextet for methylene at δ 1.77 and triplet at δ 2.68 for another methylene. Compound 2g, in its IR spectrum exhibited band at 1651cm-1 for C=O group. PMR spectrum showed singlet at δ 7.45 for 1H due to olefinic proton and a doublet at δ8.18 for 1H with J = 5.6 Hz for pyridine ring proton. Compound 3d, exhibited a band due to carbonyl group at 1681 cm-1. PMR spectrum of 3d showed the signals at δ 1.01 triplet for 3H with J =7.4 Hz, δ1.75 sextet for 2H with J = 7.4 Hz and δ 2.66 triplet for 2H with J = 7.4 Hz corresponding to propyl group. Signals at δ 7.57, dd, 1H, J =1.0 & 3.6 Hz, δ 7.72 dd, 1H, J = 1.0 & 5.1 Hz are due to thiophene ring. Olefinic proton is observed at δ 7.78 as a singlet. Singlet at δ 9.74 is due to proton on pyrazole ring. Compound 4a, showed band due to carbonyl group at 1687cm-1. PMR spectrum of the compound exhibited three singlets at δ 2.09, 2.35 and 2.45 due to three methyl groups. Singlets at δ 7.82 and 10.23 are due to olefinic proton and pyrazole ring proton respectively.
Antimicrobial Activity
The antimicrobial activity of synthesized compounds 1a-h, 2a-g, 3a-e and 4a-e was determined in vitro against three bacterial strains Bacillus subtilis, Escherichia coli, Salmonella typhi and two fungal strains Alternaria alternataand F. Oxisporum. The antimicrobial activity of compounds against test fungi and bacteria by using well diffusion technique. Ciprofloxacin was used as reference compound for bacteria and ketoconazole for fungi. All the experiments were performed in triplicate.
From seen activities for tested micro-organisms are not observed for our compounds.
References
- Kullampalayam, K. S.; Aiyalu, R.; Palaniappan, S.; Wattamwar, P. P. Bioorg. Med. Chem.Lett. 2014, 24 (13), 2940-2944.
CrossRef - Evstropov, A. M.; Yavorovskaya, V. E.; Vorobev, E. S.; Khudonogova, Z. P.; Medvedeva, S. G; Filmonov, V. D.; Prishchep, T. P.; Saratikov, A. S.J. Pharma. Chem. 1992, 26, 426-430.
CrossRef - Al-Haiza, M. A.; El-Assiery, S. A.; Sayed, G. H.Acta. Pharma. 2001, 51, 251-261.
- Sivakumar, K. K.; Rajashekhran, A.; Bugreddy, S. Int. J. Res. Pharma. Chem. 2012, 2, 327-337.
- Devnath, H. P.; Islam, M. R. Banladesh J. Pharmacol. 2010, 5, 30-33.
CrossRef - Ragab, F. A.; Abdel-Gawad, N. M.; Georgey, H. H.; Said, M. F. Chem. Pharma. Bull. 2013, 61(8), 834-845.
CrossRef - Soni, J. P.; Sen, D. J.; Moth, K. M. J. Appl. Pharma. Sci. 2011, 1, 115-120.
- Ye, D.; Zhang, Y.; Wang, F.; Zhang, M.; Zhang, X.; Jiang, H.; Liu, H. Bioorg. Med. Chem. 2010, 18, 1773-1782.
CrossRef - Bell, F. W.; Cantrell, A. S.; Hogberg, M.; Jaskunas, S. R.; Johansson, N. G.; Jordan, C. L.; Kinnick, M. D.; Lind, P.; Morin, J. R.; Noreen, R.; Oberg, B.; Palkowitz, J. A.; Parrish, C. A.; Pranc, P.; Sahlberg, C.;, Ternansky, R. J.; Vasileff, R. T.; Vrang, L.; West, S. J; Zhang, H.; Zhou, X. X. J. Med. Chem. 1995, 38, 4929-4936.
CrossRef - Patt, W. C.; Hamilton, H. W.; Taylor, M. D.; Ryan, M. J.; Taylor, Jr D. G.; Conolly, C. J. C.; Doherty, A. M.; Klutchko, S. R.; Sircar, I.; Steinbaugh, B. A.; Batley, B. L.; Painchaud, C. A.; Rapundalo, S. T.; Michniewicz, B. M.; Olson, S. C. J. Med. Chem. 1992,35, 2562-2572.
CrossRef - Rudolph, J.; Theis, H.; Hanke, R.; Endermann, R.; Johannsen, L.; Geschke, F. U. J. Med. Chem. 2001, 44, 619-626.
CrossRef - Yerragunta, V.; Suman, D.; Swamy, K.; Anusha, V.; Patil, P.; Naresh, M. Pharma Tutor. 2014, 2(1), 40-48.
- Mu, J-X.; Shi, Y-X.; Yang, M-Y.; Sun, Z-H.; Liu, X-H.; Li, B-J.; Sun, N-B.; Molecules, 2016, 21, 3-11.
CrossRef - Ouyang, G.; Chen, Z.; Cai, X-J.; Song,B-A.; Bhadury, P. S.; Yang, S.; Jin, L-H.; Xue, W.; Hu, D-Y.; Zeng,S. Bioorg. Med. Chem. 2008, 16, 9699-9707.
CrossRef - Domiai, S.; El-Mallah, A.; Ghoneim, A.; Bekhit, A.; Razik, H. A. E. Inflammopharmacol. 2016, 24, 163-172.
CrossRef - Kuettel, S.; Zambon, A.; Kaiser, M.; Burn, R.; Scapozza, L.; Perozzo, R. J. Med. Chem. 2007, 50, 5833-5839.
CrossRef - Farag, A. M.; Mayhoub A. S.; Barakat, S. E.; Bayomi, A.; H. Bioorg. Med. Chem. 2008, 16, 881-889.
CrossRef - Patel, N. B.; Agravt, S. N.; Shaikh, F. M. Med. Chem. Res. 2016, 20, 1033-1041.
CrossRef - Bassyouni, F. A.; Tawfik, H. A.; Soliman, A. M.; Rehim, M. A. Res. Chem.Intermed. 2012, 38, 1291-1310.
CrossRef - Fadda, A .A.; Berghot, M. A.; Amer, F. A.; Badawy, D. S.; Bayoumy, N. M. Arch. Pharm. Chem. 2012, 345(5), 378-385.
CrossRef - Vijesh, A.M.; Isloor , A. M.; Telkar, S.; Arulmoli, T.; Fun, H-K. Arebian J. Chem. 2013, 6(2), 197-204.
- Fascio, M. L.; Errea, M. I.; D’Accorso, N. B. Eur. J. Med. Chem. 2015, 90, 666-683.
CrossRef - Steenackers, H. P.; Levin, J. C.; Janssens, J. C.; Weerdt, A. D.; Balzarini, J.; Vanderleyden, J.; De Vos, D. E.; De Keersmaecker, S. C. Bioorg. Med. Chem. 2010, 18, 5224-5233.
CrossRef - Takate, S. J.; Karale, B. K.; Salve, S. P.; Zaware, B. H.; Jadhav, S. S. Orient. J. Chem. 2015, 31(1), 307-315.
CrossRef - Zhang, Y.; Zheng, K.; Yan, H.; Jin, G.; Shao, C.; Zhou, X.; Zhou, Y.; He, T. 2014, http://www.biomedcentral.com/1471-230X/14/62.
- Sachdeva, H.; Dwivedi, D.; Goyal, P. Org. Chem. Int. 2013, Article ID976032, http://dx.doi.org/10.1155/2013/976032.
CrossRef - Madje, B. R.; Shindalkar, S. S.; Ware, M. N. Shingare, M. S. Arkivoc(xiv) 2005, 82-86.
- Gadakh, A.; Pandit, C.; Rindhe, S. S.; Karale, B. K. Int. J. Chem. 2(2), 2010, 206-212.
CrossRef - Gadhave, A.; Kuchekar, S.; Karale, B. K. J. Chem. 2013, doi. org/10.1155/2013/741953.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









