Investigation of Langmuir and Freundlich Adsorption Isotherm of Co2+ Ion by Micro Powder of Cedar Leaf
Gh. Haghdoost, H. Aghaie and M Monajjemi
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: H.aghaie1@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330363
In this paper, the micro powder was the product of cedar leaf (MPCL) is used as a low-cost and natural adsorbent for the removal of Co2+ from aqueous solutions. Factors such as the pH effect initial metal ion concentration, contact time, MPCL dosage and temperature on the adsorption performance of MPCL for Co2+ ions were experimented by batch method. Results indicate that removal of Co2+ ions at pH values of 7, the Langmuir isotherm model is the most suitable one for the adsorption process using MPCL (R2=0.9775), thus indicating the applicability of monolayer coverage of Co (II) ion on MPCL surface. The Relationship between Thermodynamic parameters was used to predict the absorption process. According to Thermodynamic analysis, the process exothermic and natural.
KEYWORDS:Co2+; adsorption; micro powder; cedar leaf; Thermodynamic; MPCL
Download this article as:| Copy the following to cite this article: Haghdoost G, Aghaie H, Monajjemi M.Investigation of Langmuir and Freundlich Adsorption Isotherm of Co2+ Ion by Micro Powder of Cedar Leaf. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Haghdoost G, Aghaie H, Monajjemi M.Investigation of Langmuir and Freundlich Adsorption Isotherm of Co2+ Ion by Micro Powder of Cedar Leaf. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33730 |
Introductoin
Toxic heavy metals pollution is one of the influential environmental problems, because they are Non degradable and harmful for public health, even at very low concentrations. Cobalt is one of several commonly occurring toxic metals. It is an animal carcinogen producing cancer at various sites. Exposure to cobalt is extremely irritating to the skin both on contact and by provoking an allergic reaction which sensitizes the skin to further contact. It is also irritating to the eyes and mucous membrane, causing severe discomfort in the nose, often leading to perforation of the nasal septum. The threshold limit value for cobalt fume and dust exposures is 0.1 mg/m3 in the U.S. The use of activated carbons to remove cobalt from water was proposed because of their high surface areas and active functional groups leading to a search for low-cost adsorbents in recent years 1-5.
Indeed agricultural waste for example, has two advantages. First, waste material is converted to useful, value-added adsorbents. Disposal of agricultural by-products has become a major costly waste disposal problem. Second, produced activated carbons are used for removing organic chemicals and metals from wastewater 6-9. Corn cob, flamboyant pods, apricot stone, almond shell, nut shell, peach stone, oat hulls, coconut husk, coconut shell, hazelnut shell, grape seed, olive stone and Rosa cantina sp. Seeds 10, 11 . The fruit has a sweet edible pulp, the leaves are applied locally to sores, and the roots are used to cure and prevent skin diseases 12. In the present study, MPCL prepared used as an adsorbent to remove cobalt from aqueous solutions. The adsorption of cobalt ions onto MPCL was studied in batch equilibrium conditions.
Experimental
Apparatus and Materials
An AA 680 model atomic absorption spectrometer (Shimadzu Co.) was used for measuring the concentration of Co2+ion in studied solutions, a 820 A model pH meter (Metrohm Co.) was used to measure pH of solutions and a thermostatic orbit incubator shaker neolab model (India) was used to measure contact time in solution. All chemical materials used in this study were of analytical grade. MPCL prepared by chemical activation with KOH was characterized. Cobalt nitrate, was purchased from Merck Company.
Batch Adsorption Experiments
Batch adsorption experiments were carried out to determine the Co2+ ions adsorption isotherm onto MPCL and its thermodynamic properties. Co2+ ions stock solution (100 mg. L-1) was prepared by dissolving the appropriate quantity of Co (NO3)2 salt in deionized water. Adsorption isotherms were obtained by using initial Co2+ion concentration, Co, and its equilibrium concentration, Xe, at 298 K. The effect of pH on the Co2+ ions adsorption onto MPCL was conducted in a pH range of 3-10. The pH of solutions was adjusted by 0.1 M HCl or 0.1 M NaOH solutions. For every experiment, 100 ml of the solution with Co2+ concentration of 30 mg. L-1 was mixed with 50 mg of MPCL in a 250 ml glass conical flask. The flask was shaken in a thermostatic orbit shaker at 220 rpm for 60 min. The mixed was filtered through a 0.45 μm membrane filter. The filtrate was measured by atomic absorption then, the adsorption percentage (%A) was determined as:
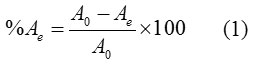
The amount qe (mg g-1) of Co2+ adsorbed at equilibrium was calculated using the following equation:
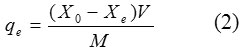
Where M is the mass of MPCL (g) , V is the volume of the solution (L) , Xo and Xe are the initial and final concentration of Co2+ ion in solution (mg L-1) respectively.
To evaluate the thermodynamic properties of the adsorption process, 40 mg of MPCL was added into the 100 ml solution with pH of 7.0 and initial Co2+ concentration ranging from 30 mg. L-1 in every experiment. Each solution was shaken continuously for 60 min at 298 K 13.
Then Langmuir and Freundlich models were fitted on experimental data. The Freundlich isotherm is appropriate to both monolayer and multilayer adsorption and is based on the assumption that the adsorbates are adsorbed onto the heterogeneous surface of an adsorbent 14. The Langmuir isotherm assumes monolayer adsorption on a uniform surface with a finite number of adsorption sites 15.
The essential characteristic separation constant factor, RL, for the Langmuir adsorption is defined as follows:

The value of RL illustrate the shape of the isotherm to be either unfavorable (RL>1), linear (RL=1), favorable (0<RL<1) or irreversible (RL=0).
The equations and parameters of isotherms of this research have been presented in Tables 1 and 2, respectively.
 |
Table 1: Equation model used in this study |
Table 2: Model parameters used in this study
| Coefficient | Description |
| KF | Freundlich isotherm constant (dimensionless) |
| n | Freundlich isotherm exponent (dimensionless) |
| KL | Langmuir constant (L mg-1) |
| qm | maximum adsorption capacity (mg g−1) |
Results and Discusston
Adsorption Study
The Effect of pH
Solution pH is one of the most important parameters to determine the adsorption property of an adsorbent that it controlled the kind and amount surface charge of the adsorbent 16, 17. Table 3 illustrate the effect of the pH of the solution on the adsorption percentage of Co2+ ion adsorbed onto MPCL. The adsorption percentage was increased with pH and optimum pH was 7.0.
The Effect of Dosage
The effect of MPCL dosage on the adsorption percentage of Co2+ ion are shown in table 3. We concluded that the dosage of 40 mg of MPCL was the most suitable. After optimum dosage, all active sites are entirely exposed and the adsorption on the surface is saturated.
The Effect of Temperature
Table 3 show that the adsorption percentage decrease with increasing temperature. Therefore, it may be concluded that the interaction between Co2+ ions and MPCL is exothermic in nature. Adsorption decrease may be due to increase the electrostatic repulsion between of the Co2+ ions.
The Effect of Contact Time
The effect of contact time, tc , on the adsorption percentage of Co2+ ion onto MPCL are shown in table 3. It becomes slower as the adsorbed amount of Co2+ ion reaches its equilibrium value. It can be seen that the adsorption process is rapid due to the availability of very active sites on the adsorbent surface at initial stage. This may be due to the special one atom layered structure of MPCL 18. The optimum contact time was obtained as 50 min.
Table 3: The effect of pH (3-7), MPCL Dosage (10-70 mg), temperature (298-348 K) and Contact Time (10-80 min) on Co2+ adsorbed by MPCL.
| pH | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Co2+Removal% | 71.2 | 73.2 | 75 | 76 | 77.6 | 76 | 75.8 | 75.5 |
| MPCL Dosage(mg) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | – |
| Co2+Removal% | 56.2 | 63.6 | 78.1 | 78.3 | 78.3 | 78.3 | 78.3 | – |
| Temperature(K) | 298 | 308 | 318 | 328 | 338 | 348 | – | – |
| Co2+Removal% | 89.6 | 82 | 78.1 | 76.2 | 70.5 | 66.4 | – | – |
| Time(min) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| Co2+Removal% | 42 | 53 | 57.2 | 67.4 | 76 | 76 | 76 | 76 |
Adsorption Isotherm
An adsorption isotherm is characterized by certain constant values, which express the surface properties of the adsorbent and so on the percentages adsorption of Co2+ ion MPCL as a function of initial concentration of Co2+ ions are given in table 4.
Table 4: Adsorption data for Co2+ions adsorption onto MPCL (pH = 7, tc=50 min, T=298 K, MMPCL =40 mg)
| Parameter | Value | |||||
| X0 /mg L-1 | 10 | 20 | 30 | 40 | 50 | 60 |
| %A | 54 | 64 | 74.6 | 80 | 83.34 | 85.73 |
| Xe / mg L-1 | 4.6 | 6.2 | 7.62 | 8 | 8.33 | 8.562 |
| qe / mg g-1 | 13.5 | 32 | 55.95 | 80 | 104.17 | 128.595 |
| log Xe | 0.663 | 0.86 | 0.882 | 0.903 | 0.921 | 0.933 |
| log qe | 1.13 | 1.51 | 1.75 | 1.9 | 2.02 | 2.11 |
| 1/Xe /L mg-1 | 0.22 | 0.139 | 0.131 | 0.125 | 0.12 | 0.117 |
| 1/qe /g mg-1 | 0.0714 | 0.03125 | 0.0179 | 0.0125 | 0.0096 | 0.00778 |
The plots of two-mentioned Langmuir and Freundlich models are shown in Figs 1 and 2.
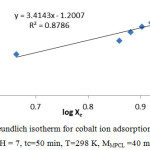 |
Figure 1: Freundlich isotherm for cobalt ion adsorption onto MPCL (pH = 7, tc=50 min, T=298 K, MMPCL =40 mg).
|
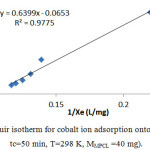 |
Figure 2: Langmuir isotherm for cobalt ion adsorption onto MPCL (pH = 7, tc=50 min, T=298 K, MMPCL =40 mg). |
The isotherm parameters calculated from the Langmuir and Freundlich models are listed in Table 5.
Table 5: The resultant values for the studied isotherms in connection to Co2+ions adsorption onto MPCL (pH = 7, tc=50 min, T=298 K, MMPCL =40 mg)
| Isotherm | Parameter | Value |
| Freundlich | KF /(L g-1) | 15.9 |
| n | 0.3 | |
| R2 | 0.8786 | |
| Langmuir | KL / (L mg-1) | 0.102 |
| qm /(mg g-1) | 15.31 | |
| R2 | 0.9775 |
The calculated RL values versus initial Co2+ concentration are given in table 6, indicating that the Langmuir adsorption of Co2+ onto MPCL is favorable 19, 20.
Table 6: Separation factor for the adsorption of Co2+ onto MPCL in terms of initial concentration of Co2+ions (pH = 7, tc=50 min, T=298 K, MMPCL =40 mg)
| Xo / mg L-1 | 10 | 20 | 30 | 40 | 50 | 60 |
| RL | 0.495 | 0.33 | 0.25 | 0.2 | 0.164 | 0.14 |
Thermodynamic Parameters
The thermodynamic parameters of adsorption process can be determined from the variation of thermodynamic equilibrium constant, Ko 22-27. Where Ko and adsorption standard free energy change (ΔG0) is calculated according to:
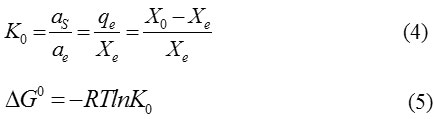
Where αs and αe are the activity of adsorbed Co2+ and the activity of Co2+ in solution at equilibrium, respectively.
The average standard enthalpy change (ΔHo) and the average standard entropy change (ΔSo) are obtained from the plot of equation (6):

In order to obtain the values of ΔHo and ΔSo, was plotted lnKo against 1/T (table 7, fig. 3).
Table 7: The effect of temperature on Ko values (Xo=30 mg. L-1, pH=7, MMPCL =40 mg, tc=50 min)
|
T/K |
%A |
K0 |
|
298 |
90 |
8.62 |
|
308 |
82 |
4.56 |
|
318 |
78 |
3.57 |
|
328 |
76.3 |
3.20 |
|
338 |
71 |
2.39 |
|
348 |
66 |
1.98 |
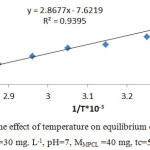 |
Figure 3: The effect of temperature on equilibrium constant values (Xo=30 mg. L-1, pH=7, MMPCL =40 mg, tc=50 min). |
The obtained values of thermodynamic parameters (ΔG0, ΔH0 and ΔS0) are listed in table 8.
Table 8: Thermodynamic parameters for adsorption Co2+ ions onto MPCL(Xo=30 mg. L-1, pH=7, MMPCL =40 mg, tc=50 min)
|
T /K |
ΔGo/kJmol-1 |
ΔHo/ kJmol-1 |
ΔSo / kJmol-1 K-1 |
|
298 |
-5.337 |
-23.84 |
-63.4 |
|
308 |
-3.885 |
-23.84 |
-63.4 |
|
318 |
-3.365 |
-23.84 |
-63.4 |
|
328 |
-3.172 |
-23.84 |
-63.4 |
|
338 |
-2.448 |
-23.84 |
-63.4 |
|
348 |
-1.976 |
-23.84 |
-63.4 |
Conclusion
The experimental data can be fitted with the Langmuir isotherm, thus indicating the applicability of monolayer coverage of Co2+ ion on MPCL surface. The calculated RL values versus initial Co2+ concentration that the Langmuir adsorption of Co2+ onto MPCL is favorable
Thermodynamic analysis showed that the adsorption process is exothermic and spontaneous in nature. Negative value of ΔH0 suggests that the interaction of adsorbed Co2+ with MPCL is an exothermic process, which is supported by the decreasing the amount of cobalt ion adsorption with increasing temperature. The negative value of ΔS0 indicates a decrease randomness and mobility at the adsorbent/solution interface during the adsorption of cobalt ion onto MPCL. The negative values of ΔG0 reveals the fact that the adsorption process is spontaneous.
References
- Mukharjee, A.G. Envirinmental pollution and health hazards; causes and control, in: S. Galgotia (ed.), New Delhi. 1986, 58.
- Dara, S.S. A text book of environmental chemistry and pollution control, in: S.Chand and Company Ltd (ed.), New Delhi. 2002, 215.
- Goel, J., Kadirvelu, K., Rajagopal, C., Garge, V.K. Journal of Hazardous Materials. 2002,125,211-220.
CrossRef - Netzer, A., Hughes, D.E. Water Research. 1984, 18, 927-933.
CrossRef - Kadirvelu, K., kavipriya, M., Karthika, C., Radhika, M., Vennilamani, N., Pattabhi, S. Bioresour Technol. 2003, 87, 129-132.
CrossRef - Vijayaraghavan, K., Winnie, H.Y.N., Balasubramanian, R. Desalination. 2011, 266, 195-200.
CrossRef - Abdul Halim, A., Abdul Aziz, H., Johari, M.A.M., Ariffin, K.S. Desalination. 2010, 262, 31-35.
CrossRef - Kilic, M., Apaydin-Varol, E., Putun, A.E. Journal of Hazardous Materials. 2011, 189, 397-403.
CrossRef - Matos, J., Nahas, C., Rojas, L., Rosales, M. Journal of Hazardous Materials. 2011, 196, 360-369.
CrossRef - Bazargan-Lari, R., Bahrololoom, M.E., Nemati, A. Journal of Food, Agriculture & Environment. 2011, 9, 892-899.
- Mozammel, H.M., Masahiro, O., Bahattacharya, S.C. Biomass and Bioenergy. 2010, 22, 397-400.
CrossRef - George, Z.K. Journal of Hazardous Materials. 2012, 5, 1826-1840.
- Demiral, H., Demiral, I., Karabacakoglu, B., Tumsek, F. Chem. Eng. Res. Des. 2011, 89, 206-213.
CrossRef - Sirajudeen, J., Naveen, J., Arul Manikandan, S., Mohamed Mubashir, M.M. Chem. Sin. 2013, 4(2), 133–143.
- Boparai, H.K., Joseph, M., Carroll, D.O. J. Hazard. Mater. 2011, 186, 458–465.
CrossRef - Yimenez-Reyes, M., Solache-Rios, M. J. Hazard, Mater. 2010, 180, 297-302.
CrossRef - Wu, Y., Zhang, S., Guo, X., Huang, H. Bioresour, Technol. 2008, 99, 7709- 7715.
CrossRef - Yang, C.H. J. Colloid interface Sci. 1998, 208, 379-387.
- Niwas, R., Gupta, U., Khan, A.A., Vavshney, K.G. Colloids surf. A. 2000, 164, 115-119.
CrossRef - Hutson, N.D., Yang, R.T. Adsorption. 1997, 3, 189-195
CrossRef - Tan, C.Q., Xiao, D. J. Hazard. Mater. 2009,164, 1359- 1363.
CrossRef - Barkat, M., Nibou, D., Chearouche,P., Mellah, A. Chem. Eng. Process. 2009, 48, 38-47.
CrossRef - Mohammadkhani, S.; Gholami, M.R.; M. Aghaie. Orient. J. Chem. 2016, 32(1), 591-599.
CrossRef - Hashemian, S.; Parsaei, Y. Orient. J. Chem. 2015, 31(1), 177-184.
CrossRef
- Haghdoost, Gh.A. Orient. J. Chem. 2016, 32(3), 1485-1492.
CrossRef
- Ozdes, D., Gundogdu, A., Kemer, B., Duran, C., Senturk, H.B., Soylak, M. Journal of Hazardous Materials. 2009,166 (2-3), 1480-1487.
CrossRef - Sari, A., Mendil, D., Tuzen, M., Soylak, M. Journal of Hazardous Materials. 2009, 162(2-3), 874-879.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









