Synthes and Green Metric Evaluation of 2-(Chloromethyl)-3-Methyl-4-(Methylsulfonyl)Pyridine
Rohidas Gilbile1,2, Ram Bhavani2 and Ritu Vyas1
1Department of Chemistry, Pacific Academy of Higher Education and Research, Udaipur-313 024, Rajasthan, India.
2Green Evolution Laboratories, Wangapally Village, Nalgonda, 500 085, Telangana State, India.
Corresponding Author E-mail: rohidasgilbile2017@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330244
Article Received on : arch 14, 2017
Article Accepted on : April 12, 2017
2-[[(2-pyridinyl) methyl] sulfinyl]-1H-benzimidazoles are the prominent motif’s that belong to the class of prazoles. These are used in the treatment of gastroesophageal reflux disease (GERD) ulcers and other gastric acid related diseases. The present article describes the modified synthesis of 2-chloromethyl-4-methanesulfonyl-3-methyl pyridine (an intermediate utilized in the synthesis of Dexlansoprazole). The advantages of this modification involves (i) N-oxidation of 2,3-lutidine with catalytic quantity of RuCl3 in presence of oxygen (ii) One pot synthesis of 2,3-dimethyl-4-(methylthio)pyridine-N-oxide using 30% NaSH, methyl iodide and tetra butyl ammonium hydroxide (iii) Oxidation of methythio pyridine–N-oxide with 30% H2O2 followed by N-deoxygenation with RuCl3.H2O to produce 2,3-dimethyl-4-(methylsulfonyl)pyridine (iv) Chlorination of the penultimate step using trichloroisocyanuric acid to obtain the desired 2-chloromethyl-4-methanesulfonyl-3-methyl pyridine. Furthermore, green metrics assessment was calculated for the above modified scheme based on the parameters viz., atom economy (AE), reaction mass efficiency (RME) and E-factor. It was observed that, in case of step 4 (oxidation of thiomethyl pyridine-N-oxide), the E-factor value is very less 3.2 which is indicative of less waste generation, when compared to the various steps involved in the synthesis.
KEYWORDS:Atom economy; RME; E-Factor; Green metrics; Prazoles; Synthesis
Download this article as:| Copy the following to cite this article: Gilbile R, Bhavani R, Vyas R. Synthes and Green Metric Evaluation of 2-(Chloromethyl)-3-Methyl-4-(Methylsulfonyl)Pyridine. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Gilbile R, Bhavani R, Vyas R. Synthes and Green Metric Evaluation of 2-(Chloromethyl)-3-Methyl-4-(Methylsulfonyl)Pyridine. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31773 |
Introduction
A prominent class of drugs that bring out distinctive and enduring pharmacological effect by reducing the gastric acid production are the Proton pump inhibitors (PPIs). Due to their outstanding efficacy and safety, these drugs gets included in list of the most expansively marketed drugs in the world. 2-[[(2-pyridinyl) methyl] sulfinyl]-1H-benzimidazoles are the prominent motif’s that belong to the class of prazoles. These are used in the treatment of gastroesophageal reflux disease (GERD) ulcers and other gastric acid related diseases.1 Some of the examples of the proton pump inhibitors with pyridine ring nucleus (Figure 1) that are the top selling classes of pharmaceuticals that have populated this area2 are omeprazole (1, Losec), rabeprazole (3, Aciphex), pantoprazole (2, Protonix), lansoprazole (4, Prevacid) and dexlansoprazole (5). All these PPI drugs (API’s) contain the distinguishing benzimidazole unit flanked with a sulfoxide substituent at the 2-position.
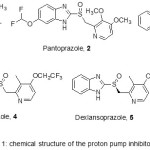 |
Figure 1: chemical structure of the proton pump inhibitors 1-5. |
A new inclusion to the proton pump inhibitor (PPI) class is Lansoprazole, which is acceptable for the treatment of heartburn related with non-erosive gastroesophageal reflux disease (GERD) and remedial for all grades of erosive esophagitis (EE) 3,4. Another prominent class of PPI is the ‘Dexlansoprazole (dex)’, it is a dextrorotatory enantiomer of lansoprazole that was developed by Takeda Pharmaceutical Co., Ltd and got approved in 2009 by US Food and Drug Administration (FDA). Later on this drug (dex) was accepted by Canada and Mexico in 2010 and 2011, respectively5. In the clinical administration, Dex exhibited lower elimination rate, excellent dominance in higher efficacy and less side effects than S-(-)- lansoprazole (levo)6.
Evaluation of the greenness of chemical processes is the outmost significance in green chemistry. In general, it is well known that in order to measure the efficiency of the process, it has to be first optimized and controlled. Managing the process in green chemistry should be implicit as a choice to select the greenest option. The improvement and application of measurable procedures allows us to differentiate the greenness of existing solutions with newly developed ones.
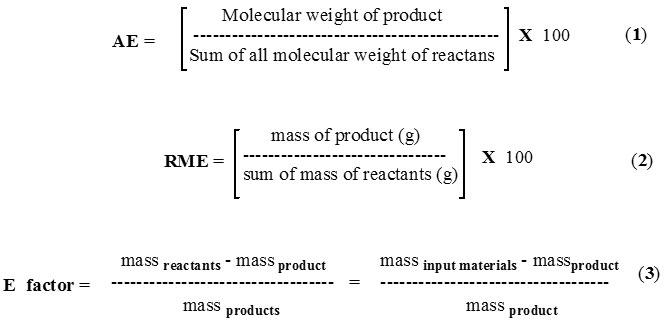
In the early 1990s, U.S. Environmental Protection Agency, has introduced the concept of green chemistry which is defined as ‘‘the utilization of a set of principles that minimizes or eliminates the use or production of unsafe substances in the design, manufacture and application of chemical products’’. An integrated set of twelve principles was recognized in order to explicate ways to extend environmentally friendly chemistry 7-10 along with sustainable chemistry metrics. 11-12 Thus the concept of atom economy paved the way to green chemistry by emphasizing the significance of the incorporation of all the atoms in the reaction product13. Atom economy (molecular weight ratio of the final product divided by the sum of all reactants, Eq.1) is not solely sufficient to determine the material economy of the reaction, though it useful to choose a synthetic pathway. A precise method to evaluate the material economy is the reaction mass efficiency (RME) which is the percentage of the mass of the reactants that remains in the product (Eq.2)14, 15. It is understood that in a chemical reaction/process, the formation of the product requires, use of reactants along with the additional required materials such as solvents, catalysts and acids and bases used in the work-up. These are the essential material in the process, though these materials does not appear in the balanced chemical equation. The Sheldon E-factor, 16 which are defined according to equation (3), respectively, permits a best measurement of the material economy. The calculation of E factor is solely depends on mass (E factor) and presents a global standpoint and is calculated as the ratio of the total mass of all waste to the mass of the desired product16. During the E-factor calculations, apart from considering the amount of the by-products (i.e amount of waste), it also take into consideration of the amount of (i) non-reacting starting materials, (ii) auxiliaries, (iii) catalysts or any additives such as acids, bases, salts, (iv) solvents of the reaction or solvents required in the work-up (extraction, washing, separation, recrystallisation, chromatographic support if not recycled, etc.). Sheldon17 has suggested one exception during the calculation of E-factor, as inclusion of water in the E-factor equation leads to exceptionally high E-factor value (aqueous waste stream), it is expelled from the calculation of E-factor.
Keeping in view of the importance of prazole drugs and related pyridine intermediates, we intend to present here the modified synthesis of 2-chloromethyl-4-methanesulfonyl-3-methyl pyridine (3)18 (an intermediate utilized in the synthesis of Dexlansoprazole) and evaluate the green metrics parameters such as atom economy (AE), reaction mass efficiency (RME) and E-factor for the various steps involved in the synthesis.
Results and Discussion
The traditional method for the synthesis of 2-chlromethyl-pyridine precursor are prepared from the starting materials19,20 viz., 2,3,5-trimethyl pyridine, 2,3-dimethyl pyridine (for the synthesis of omeprazole, rabeprazole, lansoprazole and dexlansoprazole), which is nitrated in the 4th position to form the 4-nitropyridine derivative. The 4- nitro pyridine precursor is treated with sodium methoxide and acetic anhydride to generate methoxide ion which replaces the nitro group. The methyl group at the second position is converted to the acetoxymethyl group using acetic anhydride. This acetoxy methyl group in turn is hydrolysed and chlorinated using thionyl chloride to generate the 2-chloromethyl pyridine precursor.
Synthesis of 2-chloromethyl-4-methanesulfonyl-3-methyl pyridine 7, depicted in scheme-1is the modified version of the previously reported literature method18. N-oxidaton of 2,3-lutidine in presence of 5 mol% of RuCl3X3H2O21 and bubbling of oxygen in dichloromethane at room temperature for 8h yielded 2,3-Dimethyl-pyridine-N-oxide 2 in 93% yield. This method of N-oxidation of pyridine substrate catalyzed by ruthenium with molecular oxygen as the prime oxidant, resulted in the high yield under optimal reaction conditions. The advantage of this method is (i) the easy separation of the catalysts, (ii) simple workup and environmentally acceptable makes this method a convenient approach. Chlorination of N-oxide 2 was carried out by bubbling chlorine gas22 in dichloromethane at 25oC for 3h (in two equal intervals of 1.5 h) resulted in the desired 4-chloro-2,3- dimethyl-pyridine-N-oxide 3 in 49% yield. Nucleophillic substitution with NaSH followed by alkylation with MeI in presence of 40% tetra butyl ammonium hydroxide 23 resulted in the formation of 2,3-dimethyl-4-(methylthio)pyridine-N-oxide 4 in 85% yield. In this method, the aqueous tetra-n-butyl ammonium hydroxide solution (TBAOH) was applied as strong base, reaction medium and phase-transfer catalyst. Oxidation of methythio-pyridine 4 was achieved in presence of 30% H2O224 (portion wise addition in intervals of 2h, 6h and 10h) at 75oC for 24h gave 4-Methanesulfonyl-2,3-dimethyl-pyridine-1-oxide 5 in 85% yield. This method of oxidation with 30% H2O2 is measured as a highly atom-economic, solvent and catalyst free oxidation. N-deoxygenation of 5 in presence of RuCl3x3H2O25 in acetonitrile at 85oC for 1h resulted in the formation of 2,3-dimethyl-4-(methylsulfonyl)pyridine 6 in 85% yield. This method is mild, competent and avoidance of harsh reagents and is of reasonably a broad scope for the deoxygenation of N-oxides. Allylic chlorination of 6 with trichloroisocyanuric acid26 in chloroform at reflux for 1h gave the desired 2-(chloromethyl)-3-methyl-4-(methylsulfonyl)pyridine 7 in 82% yield. As trichloroisocyanuric acid (TCCA) is safe in handling and due to its efficient expulsion of chlorine (for use in reactions all three chlorine atoms are active) in the chemical process, it is used as a chlorination and oxidation reactions also on large scale. TCCA is more atom economical and is also highly soluble in organic solvents as well as more economical, when compared to N-Chlorosuccinamide (the most-used N-haloamide), thus making it the better reagent for large-scale use.
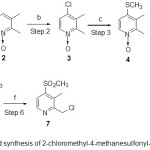 |
Scheme 1: Modified synthesis of 2-chloromethyl-4-methanesulfonyl-3-methyl pyridine 7 Click here to View scheme |
Reaction Conditions
a) RuCl3·3H2O, O2, dichlor methane, r.t., 8h; b) Cl2, dicloromethane, 25oC, 3 h; c) i. 30% NaSH, tetra-butyl ammonium hydroxide, 70oC, 5h. ii. tetra-butyl ammonium hydroxide, MeI, 15-20oC, 8h; d) 30% aq.H2O2, 75oC, 24h; e) RuCl3·3H2O, acetonitrile, 85oC, 1h; f) trichloroisocyanuric acid, chloroform, reflux, 1h;
The structural elucidation of the intermediates and the final 2-chloromethyl-4-methanesulfonyl-3-methyl pyridine 7 were characterized by the various spectroscopic techniques like 1H NMR, mass and IR data. As an example, the 1H NMR of 2-(chloromethyl)-3-methyl-4-(methylsulfonyl)pyridine 7 is described here, the protons resonating at δ 8.68 and 7.90 ppm as doublets with two proton integration is assigned to the pyridine ring protons and the proton resonating δ 4.82 ppm as singlet is assigned to the methylene proton (-CH2Cl) while the singlet signals at 3.15 ppm and 2.83 ppm is assigned to the groups –SO2CH3 and –CH3 flanked to the pyridine ring nucleus. The molecular ion of the compound in the mass spectra with m/z, 220.1 (M+1) further supports the confirmation of the desired product.
In view of the significant importance of green metrics evaluation, we have calculated the atom economy (AE), reaction mass efficiency (RME) and E-factor for the various steps that are involved in the synthesis of 2-(chloromethyl)-3-methyl-4-(methylsulfonyl)pyridine 7. The calculation of these parameters is tabulated in Table-1. In general, the % of AE is less than 100% due to the formation of various by-products involved in the individual steps, while the variation in RME is recognized to the factors such as number of reactants involved in the reaction, usage of excess molar equivalents of reactants and poor yields of the products.
Among the various steps, step 1 indicates, higher % of yield, AE and RME values i.e., 99, 97, 93% respectively, though these parameters measures the effectiveness of the reaction but in terms of E-factor value (12.48) it is less efficient. The high value of E-factor value is attributed to the usage of voluminous amounts of solvent (during work up of the reactions), purification techniques (crystallization / column chromatography) and drying agents. In case of step 4, even though the values of AE, RME and yield (74%, 62% and 85%) are at lower end when compare to step 1, the E-factor value is 3.2, the lower value of E-factor is indicative of the less waste generation when compared to step 1. Similarly, the variation of AE, RME and E-factor for the remaining steps (Table 1) is explained based on the above criteria. The E-factor value for the various steps involved in the synthesis is observed in the following increasing pattern, step 4 (E-factor-3.2) > step 1 (E-factor-12.48) > step 2 (E-factor-14.14)> step 3 (E-factor-16.35)> step 6 (E-factor-27.04)>step 5 (E-factor-28.5). Thus, the E-factor value clearly gives information on the waste generation involved in the various steps on a laboratory scale. Based on this information, a process chemist can explore to modify the technology before going further for much higher batch size.
Table 1: AE, RME and E-factor for the various steps involved in the synthesis of 2-(chloromethyl)-3-methyl-4-(methylsulfonyl)pyridine
|
Compound No |
Step |
AE% |
Yield (%) |
RME% |
* E-factor |
|
|
|
|
|
|
|
|
2 |
1 |
99 |
93 |
97 |
12.48 |
|
3 |
2 |
81 |
74 |
59 |
14.14 |
|
4 |
3 |
36 |
85 |
31 |
16.35 |
|
5 |
4 |
74 |
85 |
62 |
3.2 |
|
6 |
5 |
45 |
85 |
39 |
28.5 |
|
7 |
6 |
42 |
82 |
59 |
27.04 |
* E-factor calculation presented under experimental section
Materials and Methods
All the chemical and solvents used for the synthesis were analytical standard from Fluka or Merck. For thin-layer chromatography (TLC) analysis, E.Merck AL silica gel 60 F254 plates were utilized and spots were visualized under UV light. The mass spectrawas recorded on Agilent ion trap MS and Infrared (IR) spectra were recorded on a Perkin Elmer FT-IR spectrometer. 1H NMR spectra was recorded in CDCl3 and DMSO-d6 with a 400 MHz (Varian Mercury plus) instrument. TMS was used as an internal standard and the chemical shift values were reported in´ (ppm) and the signals were reported as s (singlet), d (doublet), dd (doublet of doublet), t (triplet), q (quartet), m (multiplet) and coupling constants are measured in Hz. Melting point (mp) determinations were performed by using Mel-temp apparatus and are uncorrected.
Experimental Section
2,3-Dimethyl-Pyridine-N-Oxide 2
To a solution containing, 2,3-lutidine 1 (5.0g, 46.66 mmol) in dichloromethane (50 mL) was added RuCl3·3H2O (0.48 g, 5 mol%) and stirred at room temperature. Oxygen was bubbled into the reaction mixture at room temperature for 8h. After the completion of the reaction (judged by T.L.C), the catalyst was filtered and the filtrate was concentrated under vacuum to obtain the crude product. The column chromatography (Stationary phase: basic alumina, Eluent: 5% Methanol/ dichloromethane) purification resulted in the pure product 2,3-dimethyl pyridine N-oxide 2. Yield: 5.34 g , 93%; Pale yellow viscous liquid; 1H NMR (400 MHz, CDCl3): δ 8.23-8.20 (m, 1H), 7.13-7.10 (m, 2H), 2.50 (s, 3H), 2.33 (s, 3H);
E-factor = 12.4; Not accounted for column chromatography;
[5g (compound 1) + 0.48 g (RuCl3.3H2O) + 66.5 g (dichloromethane) – 5.34 g (compound 2, product x yield)] / 5.34 g.
4-Chloro-2,3-Dimethyl-Pyridine-N-Oxide 3
A quantified chlorine gas (5g, 70.48 mmol) was bubbled for 1.5 h, into a stirred solution of 2,3-Lutidine-N-oxide 2 (17.18g, 140 mmol) in dichloromethane (60 mL) that was cooled to 25oC. After the conversion had reached 50% conversion (judged by T.L.C), 50% sodium hydroxide solution (11.5 g, 2.79 mol) was added slowly for 30 minutes (maintaining reaction mixture at <25°C). A second lot of chlorine gas (5g, 70.48 mmol) was again bubbled introduced for 1.5 hours at < 25°C, until the total conversion of product is attained (monitored by T.L.C). Water (25 mL) was added to the above reaction mixture and the pH was adjusted to 6.8 using sodium hydroxide solution (aq. 50%, 2.76 g). The aqueous phase was separated and extracted with dichloromethane (2 X 20 mL ). The organic layer was concentrated under reduced pressure to afford a thick residue, that was dissolved in ethylacetate (55 mL) at room temperature and stirred at 10oC for 2 h, the precipitated solids were filtered and further washed with ethylacetate (25 mL) and dried to obtain 4-chloro-2,3- dimethyl-pyridine-N-oxide (3). Yield: 10.77g, 49%.
1H NMR (300 MHz, CDCl3): δ 8.09 (d, J = 6.9 Hz, 1H), 7.26 (d, J = 4.2 Hz, 1H), 2.56 (s, 3H), 2.40 (s, 3H); ESI-MS: m/z, 158.01 (M+H)+; IR (KBr): υmax 3430, 3088, 2518, 1638, 1415, 1389, 1263, 1207, 1140, 1080, 839, 722, 632, 566, 491;
E-factor = 14.14; Not accounting for water
[17.18 g (compound 2) + 10g (Cl2) + 14.26 g (NaOH) + 133 g (dichloromethane) + 71.8 g (Ethylacetate) – 16.26 g (product x yield)] / 16.26 g
2,3-Dimethyl-4-(Methylthio)Pyridine-N-Oxide 4
A stirred mixture of 4-Chloro-2, 3-dimethyl-pyridine -1-oxide (10g), 30% aqueous sodium hydro sulphide (3 Vol, 30mL/ 53.7 g) and tetra butyl ammonium hydroxide (40% w/w, 2.5 mL/2.48g) was heated to 70°C for 5h. The reaction mixture was poured into water (25 mL) and extracted with cyclopentyl methylether (30 mL). After routine work up procedure i.e washing with water (3x 20mL), brine solution (25 mL), drying over sodium sulphate (7g), filtration and concentration yielded the crude compound (9.35g, 95% yield, 60.23 mmol). The crude compound was dissolved in tetra butyl ammonium hydroxide (50mL/49.5g, 40% w/w in water) and added methyl iodide (10.26g, 72.28 mmol) in several portion for 15 minutes at 15-20oC and continued stirring for 8h. The solids obtained during the reaction was filtered and washed with water (4 X 25 mL), dried at the pump and isolated thiomethyl-N-oxide compound. Yield: 9.15g, 85%; IR (KBr): υmax 3319, 3069, 2924, 2171, 1698, 1596, 1444, 1424, 1388, 1328, 1242, 1077, 1017, 981, 958, 820, 731, 711, 634, 567, 497 cm-1; 1H NMR (300 MHz, dmso-d6): δ 8.11 (d, J = 6.9 Hz, 1H), 7.08 (d, J = 6.9 Hz, 1H), 2.50 (s, 3H), 2.38 (s, 3H), 2.23 (s, 3H); ESI-MS: m/z, 170.01 (M+H)+;
E-factor = 16.35; Not accounting for water and brine solution
[10g (compound 3) + 53.7g (30% NaSH) + 52g (tetra-butyl ammonium hydroxide) + 10.26 g (MeI) + 25.8g (cyclopentylmethyl ether) + 7g (Na2SO4) – 9.15 g (product x yield)] / 9.15g
4-Methanesulfonyl-2, 3-Dimethyl-Pyridine-N-Oxide 5
A stirred mixture of 2,3-dimethyl-4-(methylthio)pyridine 4 (8g, 47.27 mmol) and 30% aqueous solution of H2O2 (0.32 g, 9.40 mmol) was heated to 75°C for 2 h. Another lot of 30% aqueous solution of H2O2 was added (2g, 58.80 mmol ) at room temperature and continued at the same temperature for 15 min. The reaction mixture became homogenous and additional H2O2 was added (2g, 58.80 mmol) after 10 h and 0.5g (14.7 mmol) after 6h. Total conversion of the product was attained in 24 h. After cooling the reaction mixture to room temperature, solids was thrown out which was filtered and dried at the pump to obtain 4-Methanesulfonyl-2, 3-dimethyl-pyridine-N-oxide 5. White solid; Yield: 7.98g, 85%. 1H NMR (300 MHz, CDCl3): δ 8.36 (d, J = 7.2 Hz, 1H), 7.69 (d, J = 7.2 Hz, 1H), 3.30 (s, 3H), 2.60 (s, 3H), 2.42 (s, 3H); ESI-MS: m/z, 202 (M+H)+;
E-factor = 3.2
[8g (compound 4) + 17.6g (30% H2O2) -7.98g (product x yield)] / 7.98
2,3-Dimethyl-4-(Methylsulfonyl)Pyridine 6
To a stirred solution of acetonitrile (45 mL) containing 4-Methanesulfonyl-2, 3-dimethyl-pyridine-1-oxide 5 (5g, 24.84 mmol) was added RuCl3x3H2O (5.15g, 24.82 mmol) and heated to 85oC for 1h. The completion of the reaction was judged by, the solvent was concentrated and the obtained residue was treated with water (50 mL), made alkaline with 25% aqueous ammonia (20 mL) and extracted with dichloromethane (35 mL). The dichloromethane layer was dried over Na2SO4 (8.5g), filtered and evaporated to obtain the crude product. Puirifcation of the crude product by column chromatography (silica), using (20% ethyl acetate/hexane-200 mL) resulted in the formation of desired product 2,3-dimethyl-4-(methylsulfonyl)pyridine 6. Yield: 4g, 85%.
E-factor = 28.5; Not accounting for water and column chromatography
5g (compound 5) + 5.15g (RuCl3.3H2O) + 35.37g (Acetonitrile) + 46.55g (dichlormethane) + 8.5g (Na2SO4) + 17.6g (25% Aq. Ammonia) – 4g (product x yiled)] / 4g
2-(Chloromethyl)-3-Methyl-4-(Methylsulfonyl)Pyridine 7
To a refluxing solution of CHCl3 (50 mL) containing 2,3-dimethyl-4-(methylsulfonyl)pyridine 6 (5g, 27 mmol) was added trichloroisocyanuric acid (2.82 g, 12.1 mmol) in several portions for 60 min and continued reflux for 1h. The reaction mixture was cooled and poured into an ice-water mixture (aliquots of a 50% aqueous NaOH (10 g) solution were then added to get the pH to 8.5-9.0) and the resulting solution was extracted with chloroform (25 mL). The chloroform layer was dried on Na2SO4 (6.5g), filtered, and concentrated to dryness. The residue was purified by column chromatography (silica gel, eluant; 10% Methanol in chloroform-300 mL) to afford 2-(chloromethyl)-3-methyl-4-(methylsulfonyl)pyridine 7. Light brown solid; Yield: 4.85g, 82%. 1H NMR (300 MHz, dmso-d6): δ 8.68 (d, J = 5.1 Hz, 1H), 7.90 (d, J = 5.1 Hz, 1H), 4.82 (s, 2H), 3.15 (s, 3H), 2.83 (s, 3H); ESI-MS: m/z, 220.1 (M+H)+;
E-factor = 27.04; Not accounted for column chromatography;
[5g (compound 6) + 2.82g (trichloroisocyanuric acid) + 10g (NaOH) + 111.7g (chloroform) + 6.5g (Na2SO4) – 4.85g (product x yield)] / 4.85g
Conclusion
In summary, the present article describes the modified synthesis of 2-chloromethyl-4-methanesulfonyl-3-methyl pyridine 7. The merits of the synthesis involves, N-oxidation reaction 2,3-lutidine under mild conditions using RuCl3.3H2O, one pot synthesis of 4-methylthio-pyridine-N-oxide, formation of 4-(methylsulfonyl)pyridine-N-oxide using H2O2 and chlorination reaction using trichloroisocyanuric acid. Based on the E-factor assessment, it is concluded that E-factor in step 4 (oxidation of thiomethyl pyridine-N-oxide) is 3.2, which is indicative of less waste generation, when compared to the various steps involved in the synthesis. The increasing pattern of E-factor values, “ step 4 (E-factor-3.2) > step 1 (E-factor-12.48) > step 2 (E-factor-14.14)> step 3 (E-factor-16.35)> step 6 (E-factor-27.04)>step 5 (E-factor-28.5)” clearly demonstrates that E-factor value is least in the case of step 4 while for the remaining steps it varied between 12.48-28.5. Based on this information, a process chemist can explore to modify the technology before going further for much higher batch size.
Acknowledgements
One of the authors (RDG) is thankful to the Director, Green Evolution Laboratories for his helpful suggestions and constant encouragement.
References
- Giannini, E.G.; Savarino, V.; Testa, R. Dig Dis Sci. 2006, 51, 1602.
- Sachs, G.; Shin, J. M.; Howden, C. W. Aliment. Pharmacol. Ther. 2006, 23 (Suppl. 2), 2–8.
- Caldwell, J. J Clin Pharmacol. 1992, 32, 925.
- Drayer, D.E. Clin Pharmacol Ther. 1986, 40, 125.
- Sekharan, C.B.; Rao, M.P.; Naseema, S.; Kumar, G.S.; Alekhya, B. App Sci Report. 2015, 10, 105.
- Abel, C.; Desilets, A.R.; Willett, K. Ann Pharmacother. 2010, 44, 871.
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practise, Oxford University Press, New York, 1998.
- Horwath, I. T.; Anastas, P. T. Chem. Rev. 2007, 107, 2167.
- Anastas, P. Chem. Soc. Rev. 2010, 39, 301.
- Anastas, P. T. ChemSusChem. 2009, 2, 391.
- Lapkin, A.; Constable, D. Green Chemistry Metrics, ed. Wiley, 2009.
- Garcia Calvo-Flores, F. ChemSusChem. 2009, 2, 905.
- Trost, B. M. Science. 1991, 254, 1471.
- Curzons, A. D.; Constable, D. J. C.; Mortimer, D. N.; Cunningham, V. L. Green Chem. 2001, 3, 1.
- Constable, D. J. C. ; Curzons, A. D.; Cunningham, V. L. Green Chem. 2002, 4, 521.
- Sheldon, R. A. Chem. Ind. 1992, 23, 903.
- Sheldon, R. A. Green Chem. 2007, 9, 1273.
- Saswata, L.; Lakshmana Rao, V.; Swamy, S.; Verra Narayana, B.; Parameshwar, M.; Seshadri Rao, D. US 8,513,427 B2 / 2013.
- Sharada, L.N.; Satyanarayana Reddy, G.S.; Sammaiah, B.; Sumalatha. D. Asian.J. Chem. 2013, 25, 7959.
- Venkata Madhavi, Y.; Gaikwad Nikhil Baliram. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2016, 7, 2180.
- Suman, L.; Jain Bir Sain. Chem. Commun. 2002, 1040.
- De Bode, Ronus et al From PCT Int. Appl., 2002102779, 2002, December.
- Mosstafa Kazemi, Homa Kohzadi, Omran Abdi. J. Mater. Environ. Sci. 2015, 6, 1451.
- Marjan Jereb, Green Chem. 2012,14, 3047.
- Sanjay Kumar, Anil Saini, Jagir Sandhu S. Tetrahedron Letters. 2005, 46, 8737.
- Ulf Tilstam , Hilmar Weinmann. Org. Process. Res.& Dev. 2002, 6, 384.

This work is licensed under a Creative Commons Attribution 4.0 International License.









