Study on Ion Exchange Behavior of Nuclear Grade Resin Auchliteara 9366 Chemically Degraded in Hydrogen Peroxide Medium
Ashok Narhari Patange
Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai, Maharashtra 400058.
Corresponding Author E-mail: ashokpatange78@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330255
Article Received on : January 03, 2017
Article Accepted on : March 22, 2017
The ion exchange resins are important material for study of various uni-univalent and uni-bivalent ion exchange reactions for the separation, purification and the treatment of industrial effluent’s and nuclear waste. In the current investigation attempts were done to evaluate the performance and the selectivity behavior of peroxide degraded resins viz AuchliteARA-9366 towards the Br- and I- ions from the external solution. AuchliteARA-9366 resins in chloride form were subjected to Hydrogen peroxide degradation separately on the magnetic stirrer for 24h at room temperature using 20% (6 volume) and 30% (9 volume) H2O2. The ion exchange equilibrium study of the peroxide degraded AuchliteARA-9366 resin in chloride form were done separately with KBr and KI solution of concentrations range from 0.01M to 0.1M at the temperature 30.0°, 35.0°, 40.0° and 45.0°C for 3h and the equilibrium constants (K) values for Cl-/I- and Cl-/Br- ion exchange reactions were determined. It was found that during Cl-/I- ion exchange reactions, the K values of AuchliteARA-9366 resin degraded using 20% H2O2 were found to be decreases from 37.28 x 10-2 to 15.61 x 10-2 which was higher than the K values observed for the resin degraded using 30% H2O2 which decreases from 20.53 x 10-2 to 15.54 x 10-2with rise in temperature from 30.0 - 45.0°C. Similar results were observed during Cl-/Br- ion exchange reactions for the resins AuchliteARA-9366.The equilibrium constant (K) values were found to be decreases with rise in temperature shows that both Cl-/I- and Cl-/Br- ion exchange reactions were exothermic in nature and which was further supported by negative enthalpy (∆H°) and entropy (∆S°) values. The equilibrium constant (K) values of peroxide degraded resins indicates that resins effectively oxidized with increase in the concentration of H2O2 from 20% to 30% which were also seen in SEM micrographs and FTIR spectrum of the resin. The results obtained from the present study will be helpful in understanding the effects of hydrogen peroxide degradation on the performance and halide ion selectivity behavior of ion exchange resins AuchlitARA-9366.
KEYWORDS:Hydrogen peroxide degradation; AuchliteARA-9366; anion exchanger; nuclear and industrial grade resin; enthalpy
Download this article as:| Copy the following to cite this article: Patange A. N. Study on Ion Exchange Behavior of Nuclear Grade Resin Auchliteara 9366 Chemically Degraded in Hydrogen Peroxide Medium. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Patange A. N. Study on Ion Exchange Behavior of Nuclear Grade Resin Auchliteara 9366 Chemically Degraded in Hydrogen Peroxide Medium. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=32450 |
Introduction
The AuchliteARA-9366 was a strong base nuclear grade anion exchange resin having cross linked polystyrene matrix. Strongly basic anion exchange resins can be useful for 1waste water treatment in large number of food ,chemical and bio industry whereas the anion exchange resin with benzyllic ternary ethyl or methyl ammonium groups were chemically unstable and not suitable above 60°-70°C temperatures in the hydroxide form2.Organic ion exchange resins were more versatile and selective than inorganic ion exchange resins and readily available to fulfil the demands of the various industries3.The inorganic ion exchangers were more selective towards the separations of specific radioactive ions like Cs+ and Sr+ from nuclear waste than the organic exchangers5.The inorganic exchangers6becomes more useful in nuclear industry than conventional organic resins especially in high purity water applications7.Considering the extensive industrial applications of ion exchange resin, in the present investigation attempts were made to study the performance and selectivity behavior of hydrogen peroxide (20%&30%) degraded resins AuchliteARA-93668.The scanning electron microscopy (SEM) and Fourier Transform Infrared Spectroscopy (FTIR)9were used to study the surface morphology and to recognizing the degradation pattern of the resin AuchliteARA-9366 in hydrogen peroxide medium. The above resins in chloride form were subjected to peroxide degradation using 20% (6 volume) and 30% (9 volume) H2O2 and ion exchange equilibrium studies of the resins were carried out to determine standard free energy (∆G°), entropy (∆S°), enthalpy change (∆H°) and mode of their chemical degradation.
Materials and Methods
Materials
The anion exchange resin AuchliteARA-9366 in hydroxide form was purchased from Auchtel chemical Products Ltd.,Lower Parel,Mumbai,India. The various physical and chemical properties of the resins AuchliteARA-9366 was given in Table 1
Table 1: Physico-chemical properties ofAuchliteARA-9366
|
Name of Resin |
Type of Matrix | Functional Group | Average particle Size (mm) | Percentage of Moisture |
Rage of operating temperature(°C) |
| AuchliteARA9366 | ~Polystyrene-divinyl benzene ~ | ~R4N+,OH–~ |
0.3-0.9 |
40-50 |
50-60.0 |
Chemical Degradation of Resins
The performance of chemically degraded AuchliteARA-9366 resins was studied by exposing it to hydrogen peroxide (20% H2O2&30% H2O2) medium. In order to bring about hydrogen peroxide (20% H2O2&30% H2O2) degradation, 25g of the resins in chloride form are transferred separately in 100mL round bottom flask containing 50mL of 20% H2O2 and 30% H2O2and the mixture was stirred on the magnetic stirrer for 24h. The ished chemically degraded resins in chloride form are air dried over P2O5 in desiccators and used for further studies.
Conditioning and Equilibration of Chemically Degraded Resins in Hydrogen Peroxide (20% H2O2 and 30% H2O2) Medium
In order to completely convert the resins AuchliteARA9366 in chloride form, about 80g of resin are placed in glass column 60cm long and 2.5cm in diameter10and eluted with 1L of 1M potassium chloride solution at the rate of 2mL/min.the resin is then washed with distilled water and methanol to remove excess of chloride ions and are air dried over P2O5in desiccators, stored in stoppard bottle and used for further study11. The ion exchange equilibration study of the above 20% H2O2&30% H2O2 degraded AuchliteARA9366 in chloride form were carried out separately for 3h with KBr and KI solutions12of different concentration from 0.01M, 0.025M, 0.05M, 0.075M and 0.10M in the equilibration temperatures between 30.0°C to 45.0°C as explained13.After 3 hours, the amount bromide and iodide ions [15] exchange with the resin can be determined potentiometrically14-15 by using standard 0.1M AgNO3.
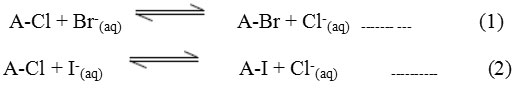
Where A=AuchliteARA-9366
From the results the equilibrium constants K for the reaction (I) and (II) were calculated in the equilibration temperature ranging from 30.0°C to 45.0°C.
Results and Discussion
The equilibrium constants (K) 16for reactions I and II were calculated by the equation
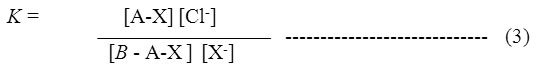
Here, A =AuchliteARA-9366 surface; B = anion exchange capacity of the resin; X–= Br– and I– ions. Thus by knowing different concentrations of X–ions in solution at a given temperature, equilibrium constant (K) values were calculated and mean K-values for this set of experiment was determined. Similar K values were calculated for the Cl–/Br– and Cl–/I– ion exchange reactions carried out at different temperatures. A graph was plotted in between log K and 1/T (0K) (figure1-3), from the slope of a graph, the standard enthalpy change ΔH°, ΔG° and ΔS° in kJ.mol-1of Cl–/Br– and Cl–/I– ion exchange reactions were determined for K-values at different temperatures. Similarly ΔH°, ΔG° and ΔS° and equilibrium constant (K), for the above Cl–/Br– and Cl–/I– ion exchange reactions were also determined for the resin subjected to degradation in hydrogen peroxide (20% H2O2&30% H2O2) medium. The thermodynamic parameters calculated for Cl–/Br– and Cl–/I– reactions of fresh resinsAuchliteARA-9366 and resins exposed to degradation in hydrogen peroxide (20% H2O2&30% H2O2) medium were represented in Tables 2 to 5.
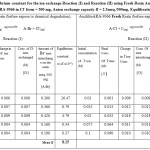 |
Table 2: Equilibrium constant for the ion exchange Reaction (I) and Reaction (II) using Fresh Resin AuchliteARA-9366.Amount of AuchliteARA-9366 in Cl– form = 500 mg, Anion exchange capacity B = 2.3meq./500mg, Equilibration temperature = 30.0°C. Click here to View table |
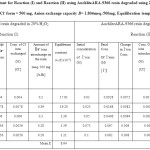 |
Table 3: Equilibrium constant for Reaction (I) and Reaction (II) using AuchliteARA-9366 resin degraded using 20% H2O2. Amount of AuchliteARA-9366 in Cl– form = 500 mg, Anion exchange capacity B= 1.804meq./500mg, Equilibration temperature = 30.0oC. Click here to View table |
 |
Table 4.Equilibrium constant for Reaction (I) and Reaction (II) using AuchliteARA-9366 resin degraded using 30% H2O2. Amount of AuchliteARA-9366 in Cl– form = 500 mg, Anion exchange capacity B= 1.78meq./500mg, Equilibration temperature = 30.0oC. Click here to View table |
 |
Table 5: Thermodynamics of Cl–/Br– and Cl–/I– reactions using fresh AuchliteARA-9366 and degraded resin in 20% and 30% H2O2medium. Click here to View table |
Auchliteara-9366 Resins Degraded in 20% H2O2 Medium
were found to decreases from 37.28×10-2 to 15.61x 10-2 and from 8.64 x10-2 to 4.97 x10-2 respectively with rise in equilibration temperature from 30.0oC to 45.0oC. The standard enthalpy change (ΔHo), (ΔGo) and (ΔSo) calculated for Cl–/I– ion exchange reactions were -42.58, 3.78 and -0.15 kJ.K-1mol-1 respectively which were less than the respective values of -32.12,6.96 and -0.12 kJ.K-1mol-1 respectively as that obtained for Cl–/Br– ion exchange reactions (Table 5).similarly (K) values for Cl–/I– and Cl–/Br– ion exchange reactions for
Auchliteara-9366 Resins Degraded in 30% H2O2 Medium
were found to decreases from 20.53×10-2 to 15.64×10-2 and from 5.94×10-2 to 4.74×10-2 respectively with rise in equilibration temperature from 30.0oC to 45.0oC. The standard enthalpy change (ΔHo) ,(ΔGo) calculated for Cl–/I– ion exchange reactions were -14.14 and 4.50 kJ.mol-1respectively which were lower than the respective values of -13.05 and 7.58 in kJ.mol-1respectively as that obtained for Cl–/Br– reactions. However, slightly large value of (ΔSo) -0.06 kJmol-1is obtained for Cl–/I– ion exchange as compared to -0.07 kJ.mol-1 obtained for Cl–/Br– ion exchange reactions (Table 5). The large K values and standard enthalpy change (ΔHo), (ΔGo) and (ΔSo) values [as given in Tables 5] obtained for Cl–/I–ion exchange reactions as compared to the Cl–/Br– ion exchange reaction for fresh and peroxide degraded AuchliteARA-9366 in 20% H2O2 and 30% H2O2 medium shows that iodide ions in the solution were more selective towards the resins AuchliteARA-9366 as compared to that of bromide ions. Similarly for Cl–/I– and Cl–/Br – reactions of chemically degraded resin AuchliteARA-9366, the K-values obtained were nearly half of equilibrium constants (K) values obtained for Cl–/I– and Cl–/Br – reactions of fresh resins AuchliteARA-9366 with rise in the concentration of hydrogen peroxide from 20% to 30%. these equilibrium constants (K) values for AuchliteARA-9366 resins decreases with increase in the concentration of hydrogen peroxide indicating that the resin oxidized effectively in peroxide medium which were clearly seen in FTIR spectrum and SEM Micrograph18of the resins under similar conditions.
Graphs
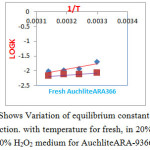 |
Figure 1: Shows Variation of equilibrium constant for Cl–/I–& Cl–/Br – reaction. with temperature for fresh, in 20% H2O2, and 30% H2O2 medium for AuchliteARA-9366. |
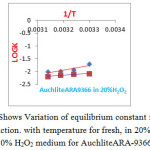 |
Figure 2: Shows Variation of equilibrium constant for Cl–/I–& Cl–/Br – reaction. with temperature for fresh, in 20% H2O2, and 30% H2O2 medium for AuchliteARA-9366. Click here to View figure |
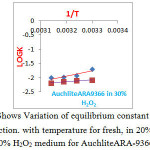 |
Figure 3: Shows Variation of equilibrium constant for Cl–/I–& Cl–/Br – reaction. with temperature for fresh, in 20% H2O2, and 30% H2O2 medium for AuchliteARA-9366. Click here to View figure |
FTIR Spectrum of Fresh and Chemically Degraded Auchliteara-9366 Resin using Hydrogen Peroxide
FTIR spectra of fresh as well as Hydrogen peroxide degraded samples of AuchliteARA-9366 resins were recorded in KBr pellets (0.002 g resin/ 0.200 g KBr) were using a PerkinElmer 1750 FTIR spectrophotometer. In the FTIR spectrum of fresh resin[19] AuchliteARA-9366, the sharp strong broad band at 3366 cm-1 corresponding to the signal vibration of O-H bond of the water or the quaternary ammonium group (R4-N+). This may be due to the moisture content of the fresh resins. The sharp band between1380-1349 cm-1is for -C-N stretching while a variable absorption bands between 1633-1614cm-1 is due to the stretching vibrations of –C=C- of alkenes group. The weak band at 3031cm-1 is the characteristic stretching band for aromatic ring. A moderate band at 2925cm-1 is due to the C-H stretching band for –CH2 group. A moderate and sharp band at 1416cm-1 and 1470cm-1 is due to the –C-H bending bands for – CH2 group. The variable band at1511cm-1 is the –C=C- stretching for aromatic ring, the sharp band at 828cm-1 and moderate band at 705cm-1 is the characteristic bands of p-substituted and o-substituted aromatic rings. On Comparing the IR spectra of fresh resin (figure 4) with the IR spectra’s of degraded resin in 20% and 30% peroxide medium (figure 5and 6) indicate the disappearance of characteristic –C-N stretching19in quaternary ammonium group band at 1349cm-1 and the characteristic Aromatic -C-H stretching band at 3031cm-1, it can be conclude that the resin is slightly oxidized in the peroxide medium and there is no significant change in structure of the resin. However, some of the resin sites are blocked or cracked for the ion exchange reaction with rise in the concentration peroxide degradation medium from 20% to 30% which can also be seen in the SEM images(figures 8- 9) of peroxide degraded resins.
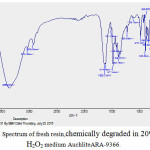 |
Figure 4: FTIR Spectrum of fresh resin,chemically degraded in 20% and 30% H2O2 medium AuchliteARA-9366.
|
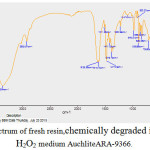 |
Figure 5: FTIR Spectrum of fresh resin,chemically degraded in 20% and 30% H2O2 medium AuchliteARA-9366. Click here to View figure |
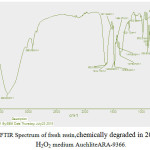 |
Figure 6: FTIR Spectrum of fresh resin,chemically degraded in 20% and 30% H2O2 medium AuchliteARA-9366. Click here to View figure |
Scanning Electron Microscopy (SEM) Studies of fresh (At Room Temperature) and Chemically Degraded Resin using 20% H2O2& 30% H2O2 Auchlite ARA-9366 Resin.
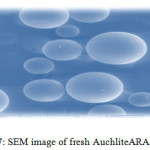 |
Figure 7: SEM image of fresh Auchlite ARA-9366 Click here to View figure |
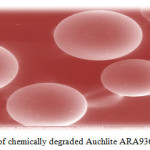 |
Figure 8: SEM of chemically degraded resin Auchlite ARA9366 in 20% H2O2 Click here to View figure |
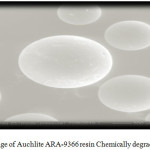 |
Figure 9: SEM image of Auchlite ARA-9366 Chemically degraded using 30% H2O2 |
Scanning electron micrographs of both fresh and degraded resin surfaces were obtained with the help of SEM technique using Scanning Electron Microscope of model no. JSM-6380LA (Jeol Ltd., Japan). The resin samples were properly placed on the aluminum sub coated double-sided graphite tape and simultaneously samples were made exposed electronically in a vacuum on thin carbon layer for 60 seconds at 30 W. The images were captured at an excitation voltage of 15 kV20under a 90 Pascal pressure and a magnification of x150, x500, x1000, x 2500 and ×5000.The SEM (Figure 7) of fresh ion exchange resins AuchliteARA-9366 shows its plane spherical structure with smooth surface. The scanning electron micrograph of chemically degraded AuchliteARA-9366 resin in 20%H2O2medium shows large hair line cracks and thread like rough appearance on the plane surface of the resin indicates the resin may be mildly oxidized in the peroxide medium (Figure 8) while SEM image of chemically degraded AuchliteARA-9366 resin in 30%H2O2medium shows heavy cracks and dots on the plane spherical surface of the resin indicates that the resin may be oxidized in the peroxide medium (Figure 9) and the surface of the resin is appearing still rough in the peroxide medium as compare to that of fresh resin (Figure 7).
Conclusion
The present research work will be helpful for understanding the degradation effect of various oxidizing and reducing agents on the performance and selectivity behavior of different industrial and nuclear grade resin materials for treatment of waste water effluents. The results and data obtained from such research work will be helpful in selecting the specific ion exchange resins for efficient industrial applications and will be useful in optimization of process parameters to achieve the maximal efficiency of the ion exchange material.
References
- Wiley, J. ; and sons. Inc. J.Appl polym Sci. 1997, 64; 1161-1167.
CrossRef - Baumann, E. ; J. Chem. and Eng. Data, 1960, 5, 376.
CrossRef - Singare P.U; Patange,A.N.; International Letters of Chemistry,Physics and Astronomy, 2014,11, 67.
- Singare P.U; Patange,A.N.;, International Letters of Chemistry,Physics and Astronomy, 2014,11, 44.
- Singare P.U; Patange,A.N.; International Letters of Chemistry,Physics and Astronomy, 2014, 6, 8.
- Singare P.U; Patange,A.N.; International Letters of Chemistry,Physics and Astronomy, 2014,6, 1.
- International atomic energy agency, Waste Treatment and Immobilization, Techniques Involving Inorganic Sorbents IAEA-TECDOC-947, Vienna 1997.
- Bhargava, A.; Janardanan,C.; Indian J.Chem.,1997,36A, 624.
- Lokhande,R.S.; Singare,P.U.; Patil ,A.; Russ. J. Phys. Chem. A,2007,81,2059.
CrossRef - Singare,P.U; Lokhande ,R.S.; Prabhavalkar, T.Bull. Chem. Soc. Ethiop.,2008 ,22:415.
CrossRef - Lokhande, R.S.;Singare, P.U.; Patil,A.B.; Radiochim. Acta,2007 95: 111.
- Lokhande,R.S.; Singare, P.U; Radiochim. Acta, 2007,95,173.
- Lokhande,R.S.;Singare,P.U.; J .Porous Mater, 2008,15, 253.
CrossRef - Hatsis,P.; Lucy, C.A.; J. Chromatogr., 2001,920A, 3.
CrossRef - McNeill,I.C.;Mohammed,M.H.; Polym. Degrad. Stab. , 1995,48,175.
CrossRef - Decker , C.; Zahouily,K.; Polym. Degrad. Stab. , 1999, 64,293.
CrossRef - Santhiya, D.; Subramanian,S.; Natarajan ,K.A.; Malghan,S.G.; J. Colloid. Int. Sci., 1999,216,143.
- Kaczmarek,H.; Felczak,A.; Szalla,A.; Polym. Degrad. Stab. ,2008, 93,1259.
CrossRef - Jiang,D.D.; Yao,Q.;McKinney,M.A.;Wilkie,C.A.; Polym. Degrad. Stab.,1999,63, 423.
CrossRef - Singare, P.U.; Ionics, 2014,20, 867.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









