Stability Indicating RP-UPLC-PDA Method Development, Validation of Multi Drug Combination of Emtricitabine, Tenofovir Alafenamide and Rilpivirine in Bulk Drug and its Tablet Formulation
Imam Pasha. S1, Murali Balaram.Varanasi1 and Ibrahim Mohammed2
1Department of Pharmaceutical Analysis and Quality Assurance, Sultan-Ul-Uloom College of Pharmacy, Banjara Hills, Hyderabad-500034, TS, India.
2Department of Analytical Chemistry, Nizam Institute of Pharmacy and Research Centre, Nalgonda, T.S, India.
Corresponding Author E-mail: imampharmaceuticalsciences@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330243
Article Received on : January 17, 2017
Article Accepted on : March 11, 2017
Stability representing Ultra Performance LC method was developed for Assay of multi drug Combination of Rilpivirine, Emtricitabine and Tenofovir alafenamide in bulk active pharmaceutical ingredients and its tablet formulation, Validation was performed for all parameters .Retention times of Reference Standard Emtricitabine, Tenofovir Alafenamide and Rilpivirine was found to be 0.965, 1.528, 2.186 with the flow rate of 0.3 mille liters per minute by Injecting Volume of 2 micro liters by maintaining Run time of 3 minutes. Developed method was subjected to forced degradation studies under specified conditions, which meets the required criteria. Degradation product at 1.975 was collected under various stress condition, degradation of imidazole ring of tenafovir alafenamide confirmed with Proton NMR, ESI–MASS in + MODE.
KEYWORDS:RP-UPLC-PDA; Emtricitabine; Tenofovir Alafenamide; Rilpivirine
Download this article as:| Copy the following to cite this article: Pasha I. S, Varanasi M. V, Mohammed I. Stability Indicating RP-UPLC-PDA Method Development, Validation of Multi Drug Combination of Emtricitabine, Tenofovir Alafenamide and Rilpivirine in Bulk Drug and its Tablet Formulation. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Pasha I. S, Varanasi M. V, Mohammed I. Stability Indicating RP-UPLC-PDA Method Development, Validation of Multi Drug Combination of Emtricitabine, Tenofovir Alafenamide and Rilpivirine in Bulk Drug and its Tablet Formulation. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31233 |
Introduction
Emtricitabine, Rilpivirine, and Tenofovir Alafenamide combination is used in the treatment of HIV-1 as initial therapy as a part of anti retroviral therapy1-3 .This combination was approved in March 2016 by US-FDA. Literature survey indicate no UPLC –PDA (stability indicating) method for this new fixed dose Alafenamide derivative combination 4-6.
As the Tenofovir disproxil fumarate causing the release of formic acid with frequent administration in HIV-I patience ,which ultimately cause GI disturbances, henceforth alternative analogue that is Tenafovir Alafenamide was proved alternative drug to that of previous analogue, The current work describes the stability indicating accurate, Precise Ultra Performance LC method for quantifying Emtricitabine, Rilpivirine, Tenofovir Alafenamide in bulk drug &its formulation by using PDA detector.
The ICH recommend stability testing for combined drug formulation for enhancing safety and to avoid the interference of degradation products during the routine analysis 7,8. The Objective of current work was to develop the method for quantifying and to study the degradation process under various stress condition for assay of long term stability testing samples and in routine analysis.
Materials and Methods
Details of Chemicals
Emtricitabine, Rilpivirine, and Tenofovir Alafenamide Standards (Aurabindo Pharma Pvt Ltd), KH2PO4 (FINE chemical LTD), Water and Methanol for UPLC (LICHROSOLV (MERCK), Acetonitrile (Batch: IA51F65129, Merck)
Instruments used
UPLC: WATERS, Aquity Empower, 2695 separation module, PDA detector
UV/VIS spectrophotometer, LABINDIA UV3000+
Weighing Machine (afcoset ER-200A))
Preparation of Diluents
Buffer
0.1M tri ethyl amine buffer was prepared, PH was adjusted to 3 with Ortho phosphoric acid and filtered through membrane filter of pore size 0.45 microns
Mobile Phase
To 35% of 0.1M tri ethyl amine buffer add 65% Acetonitrile HPLC Grade, degassed and filtered through membrane filter under vacuum condition.
Standard Solution Preparation
300ppm of Emtricitabine, 450ppm of Tenofovir Alafenamide and 37.5ppm of Rilpivirine was prepared by using the mobile phase and injected into liquid chromatography (Standard Stock Solution)
Sample Solution Preparation
Accurately weigh 10 tablets, crush and take equivalent to 200mg of Emtricitabine, 300mg of Tenofovir Alafenamide and 25mg of Rilpivirine powder sample in a 100mL volumetric flask, transfer 65 mL of mobile phase and subject to sonication for 30 minutes make up the volume with diluent. Then it is filtered through 0.45 micron filter (Sample Stock solution)
From the above take aliquots to prepare 300ppm of Emtricitabine, 450ppm of Tenofovir Alafenamide and 37.5ppm of Rilpivirine
Procedure
Inject 2 mL of the standard, sample (each six replicates) into the UPLC system and calculate the Percentage RSD and calculate the %Assay by using the formulae.
Method Validation
Precision
Procedure
The standard solution was injected for six times and measures the area for all six replicate samples in UPLC.
Intermediate Precision/Ruggedness
Procedure
The standard solutions prepared in the precision were injected on the other day, for six times and measured the area for all six samples in UPLC.
Acceptance Criteria
The % RSD for the area of six standard injections results should not be more than 2%.
Limit of Detection of Tenofovir Alafenamide
450 µg/ml, 0.18 µg/ml solutions were Prepared by taking suitable aliquots from Stock by using mobile Phase as diluents.
Calculation of S/N Ratio
Average Baseline Noise obtained from Blank: 66 µV
Signal Obtained from LOD solution: 198 µV
S/N =198/66 = 3.00
Limit of Quantification of Tenofovir Alafenamide
45 µg/ml, 0.61 µg/ml solutions were Prepared by taking suitable aliquots from Stock by using mobile Phase as diluents,degassed,filtered through membrane filter .
Calculation of S/N Ratio
Average Baseline Noise obtained from Blank: 66 µV
Signal Obtained from LOQ solution: 659µV
S/N = 659/66 = 9.98
Acceptance Criteria
S/N Ratio value shall be 10 for LOQ solution.
Linearity
From the stock solution, suitable aliquots were taken to prepare the following samples of various drug concentrations by using mobile phase as diluent
Sample– I
100ppm of Emtricitabine, 150ppm of Tenofovir Alafenamide and 12.5ppm of Rilpivirine
Sample – II
200ppm of Emtricitabine, 300ppm of Tenofovir Alafenamide and 25ppm of Rilpivirine
Sample – III
300ppm of Emtricitabine, 450ppm of Tenofovir Alafenamide and 37.5ppm of Rilpivirine
Sample – IV
400ppm of Emtricitabine, 600ppm of Tenofovir Alafenamide and 50ppm of Rilpivirine
Sample – V
500ppm of Emtricitabine, 750ppm of Tenofovir Alafenamide and 62.5ppm of Rilpivirine
Procedure
2 micro liters of each level sample was injected into liquid chromatography, peak areas were measured, calculate correlation co efficient value by plotting the graph
Degradation Studies
Hydrolytic Degradation under Acidic Condition
Transfer 1.5 ml of standard stock to volumetric flask of 10ml and add 3 ml of 0.1N Hydrochloric acid, flask was subjected to temperature of 60ºC for a period of 6 hours and the sample is neutralized with 0.1 N Sodium hydroxide and make up the volume to 10ml with mobile phase diluents, filter, inject into the system under optimized chromatographic condition
Hydrolytic Degradation under Alkaline Condition
Transfer 1.5 ml of standard stock to the volumetric flask of 10ml and add 3 ml of 0.1 N Sodium hydroxide, flask was subjected to temperature of 60ºC for a period of 6 hours and the sample is neutralized with 0.1N Hydrochloric acid and make up the volume to 10ml with mobile phase diluents, filter ,inject into the system under optimized chromatographic condition
Thermally Induced Degradation
Emtricitabine, Tenofovir Alafenamide and Rilpivirine sample was taken in Petri dish and kept in Hot air oven at 110°C for 24 hours. Then the sample was taken and diluted with diluents and injected into UPLC and analyzed.
Oxidative Degradation
Transfer 1.5 ml of standard stock to the volumetric flask of 10ml and add 1 ml of 3% w/v of hydrogen peroxide, make up the volume to the mark and keep the flask at room temperature for a period of 15 minutes, filter, inject into the system under optimized chromatographic condition
Results
Optimized Chromatographic Conditions
Temperature: Ambient
Column: Thermosil Octa Decyl Column (4.6 x 50mm, 1.7 mm)
Buffer: 0.1M Tri Ethyl Amine of PH 3.0
Mobile phase: 35% buffer 65% Acetonitrile
Rate of flow into UPLC: 0.3 mille liters per minute
Wavelength: 260 mm
Injection volume: 2 ml
Sample Run time: 3.0min
The %RSD for the area of six replicate samples was found to be within the specified limits for Precision, Intermediate Precision
Table 1: Percentage Degradation
|
Degradation aid |
Emtricitabine
|
Tenofovir Alafenamide |
Rilpivirine |
|
Standard |
0.8 |
1.2 |
1.3 |
|
Acid |
3.15 |
3.01 |
8.08 |
|
Base |
5.77 |
4.27 |
3.44 |
|
Peroxide |
4.15 |
4.97 |
8.37 |
|
Thermal |
8.07 |
6.47 |
8.74 |
|
Photo |
5.31 |
4.37 |
6.08 |
Table 2: Accuracy results for Tenofovir Alafenamide
|
%Concentration (at specification Level) |
Amount Added (mg) |
Amount Found (mg) |
% Recovery |
Mean Recovery |
|
50%(n=6) |
150 |
151.87 |
101.25 |
100.48 |
|
100%(n=6) |
300 |
301.88 |
100.63 |
|
|
150%(n=6) |
450 |
448.04 |
99.57 |
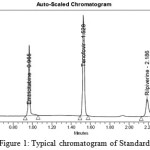 |
Figure 1: Typical chromatogram of Standard |
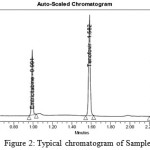 |
Figure 2: Typical chromatogram of Sample Click here to View Figure |
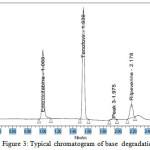 |
Figure 3: Typical chromatogram of base degradation Click here to View Figure |
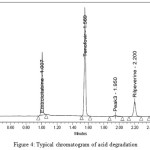 |
Figure 4: Typical chromatogram of acid degradation Click here to View Figure |
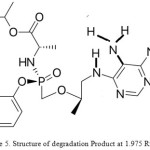 |
Figure 5: Structure of degradation Product at 1.975 Rt Click here to View Figure |
Discussion
The Method Validation is done for all parameters and the results were within the acceptance criteria, Structure of acid, base ,peroxide ,photolytic degradation product at the retention time of 1.975 is collected by using preparative UPLC , degradation of imidazole ring of tenafovir alafenamide confirmed with Proton NMR, ESI –MASS in + MODE and developed method Proved to be effective even in the presence of degradation products.
Conclusion
Forced degradation studies were performed for the developed method as per US FDA, ICH guidelines, hence proved that developed method is stability indicating, can be very effective in bulk drug synthetic sites & even in formulation premises for quantifying the amount of drug in the tablet formulation .
Acknowledgement
I convey my sincere thanks to Dr.B .Bhanu Teja, Dr.Srikanth U Allamraju, TherDose Pharma Pvt .Ltd, Hyderabad, Telangana, India, for the support and guidance for providing all the facilities by enabling me to complete this work at such a caliber.
References
- Deepthi, K.; Nagarjuna, R.G.; Dhanalakshmi, K.; I.J.O.Pharm. Research and Review. 2013, 2(10), 1-11.
- Seshachalam, U.; Haribabu,B.; Chandrasekhar, K.B.; J. O. Separation Science, 2007 30(7), 999–1004.
- Sasikala, M.; Teja, D.M.; Vinod kumar, K.; Nagamallikarjuna, R.B.; International Bulletin of Drug Research. 2013, 3(5), 20-28.
- Prabhakarreddy, A.; Chandrateja, U.; Ashrafsultan, S.K.; Vijayalakshmi, A.M.; Bachi, N. IAJPR.2014, 4(11), 5226-5234.
- Uttamprasad.P.; Sunilkumar, R.A., Der Pharmacia Lettre. 2015, 7 (1), 303-314.
- Joshi, M.; Nikalje.A.P. Shahed.M. Dehghan.M. I. J. O. Pharmaceutical Sciences. 2009, 71(1), 95–97.
- Mohan, G .B.; Lakshman, R.A.; Venkateswara, R.J.; World. J.O.Pharmacy and Pharmaceutical sciences. 2013, 4(7), 859-873.
- Prashant, S.; Devrukhakar.; Roshan, B.; Nalini, S.; Surendranath, K.V. ; ISRN Chromatography, 2013, 11, 295-303.

This work is licensed under a Creative Commons Attribution 4.0 International License.









