Kinetic Analysis of Thermal and Hydrolytic Decomposition of Spiroborate Ester of Curcumin with Salicylic Acid
Jeena John, Sudha Devi Rugmini and Balachandran Sreedharan Nair
Department of Chemistry, Mahatma Gandhi College, University of Kerala, Thiruvananthapuram, India.
Corresponding Author E-mail: sbcnair@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330234
Article Received on : March 06, 2017
Article Accepted on : April 14, 2017
Spiroborate ester of curcumin with salicylic acid (CBS) has been synthesized and characterized by different spectral techniques. The non-isothermal thermogravimetric method was used to study the thermal decomposition of CBS at the heating rate of 10 °C min-1 in nitrogen atmosphere. Hydrolytic stability was studied in aqueous acetone system with different percentages of water and temperature. The activation energies associated with the hydrolysis were evaluated using Arrhenius equation and the corresponding values of thermodynamic parameters were determined using Eyring equation. The possible mechanistic route for the hydrolysis of the complex was proposed.
KEYWORDS:Curcumin; Boric acid; Salicylic acid; Hydrolytic stability; Thermal stability
Download this article as:| Copy the following to cite this article: John J, Rugmini S. D, Nair B. S.Kinetic Analysis of Thermal and Hydrolytic Decomposition of Spiroborate Ester of Curcumin with Salicylic Acid. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: John J, Rugmini S. D, Nair B. S.Kinetic Analysis of Thermal and Hydrolytic Decomposition of Spiroborate Ester of Curcumin with Salicylic Acid. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=32083 |
Introduction
Curcuma longa commonly called as turmeric is a widely used spice, food coloring agent and preservative agent in Asian countries.1 Curcumin is the bioactive component present in turmeric and has many biological applications.2 The biological applications include anticarcinogenic,3 antioxidant,4-6 anti-inflammatory,7 anti-HIV,8 antimicrobial,9-10 anti-viral activity,10 etc. Various analogues and derivatives of curcumin have been developed to increase the bioavailability and pharmacological activities of curcumin.11-14 Among the prepared derivatives of curcumin, spiroboranes of curcumin is an important class of compounds widely used for detecting boron in various matrices in different fields like nuclear energy, metallurgy, pharmacy and agriculture.15-16 Spiroboranes of curcumin also finds application in diverse fields including material and medicinal sciences.17-18
Salicylic acid is a phenolic compound present in fruits, vegetables and spices known for its anti-inflammatory and antioxidant activity. It is the main ingredient in many skin care products.19 Curcumin is also used in skin care products because of its capability of preventing skin tumors and insults such as spots and wrinkles.20 Boron is a non-toxic, non-active and pollution free element and has many applications in the field of anticorrosion, sterilization, anti-wear and flame retardant. Commonly boric acid is used as a chemical food preservative in meat and dairy product because of its ability to inhibit the growth of microorganism. However some reports indicated that boric acid is harmful to human health if consumed in higher amount.21
Curcumin forms boron complex with salicylic acid (Scheme 1). The combination of these three biologically important compounds might be result in the formation of a compound having better biological activities. The hydrolysis and thermal stability data of this compound is necessary for the proper interpretation of its application in various fields like material and medicinal sciences. In the present study, synthesis and characterization of spiroborate ester of curcumin with salicylic acid (CBS) were discussed along with its thermal and hydrolytic stability.
Materials and Methods
Chemicals
All chemicals and solvents were of analytical grade quality and commercially available. The commercial sample of curcumin purchased from Merck Chemie Pvt. Ltd. Mumbai was further purified by column chromatography to get pure curcumin free from its derivatives.22
Measurements
UV spectra were recorded on Elico 198 Biospectrophotometer fitted with a quartz cuvette of 1 cm path length within the range of 400-700 nm. IR spectra were obtained in KBr pellet on Perkin Elmer IR spectrophotometer (4000-400 cm-1). 1H NMR and 13C NMR was recorded in DMSO-d6 solvent on Bruker Avance NMR spectrometer operating at 400 MHz. Chemical shifts was quoted in δ and were related to that of solvents. 11B NMR was recorded in 50% acetone-water system on Bruker Avance NMR spectrometer operating at 400 MHz using borax glass as reference. Thermogravimetric analyses were recorded on Perkin-Elmer Thermogravimetric Analyzer in nitrogen atmosphere at a heating rate of 10°C /min from room temperature to 700°C.
Preparation of CBS
A mixture of curcumin (0.01 M), boric acid (0.01 M), and salicylic acid (0.01 M) in 10 mL toluene were heated under reflux using a dean stark trap for 16 hrs. The reaction was monitored through TLC and after the completion of the reaction the solvent was removed through filtration to get the solid product. It was further washed with toluene to remove unreacted curcumin (Scheme 1). The structure of the compound was confirmed by different spectral techniques. The 1H NMR and 13C NMR spectrum of CBS are given in Figure 1.
 |
Figure 1: 1H NMR and 13C NMR spectrum of CBS Click here to View figure |
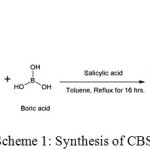 |
Scheme 1: Synthesis of CBS Click here to View scheme |
Spectral Data of CBS
Yield 83%; UV λmax= 508 nm (acetonitrile); IR (KBr) : 3449(OH), 1686 (C=O in lactone ring), 1524 (C=O in curcumin), 1287 (C-O in phenol), 1066 cm-1 (C-O in OCH3); 1H NMR (400 MHz, DMSO-d6); δ 3.83 (s, 6H, OCH3), 6.58 (s, 1H, CH), 6.85 (d, J = 8.4Hz, 2H, Ar-H), 7.33 (d, J = 10Hz, 2H, Ar-H), 7.88 (d, J = 15.6Hz, 2H, =CH), 7.03 (d, J = 15.6Hz, 2H, =CH), 6.96 – 7.85 (m, 4H, Ar), 7.46 (s, 2H, Ar-H), 10.09 (s, 2H, OH); 13C NMR: δ 55.73, 100.60, 112.64, 114.39, 115.94, 117.76, 117.98, 120.04, 125.23, 125.99, 132.24, 135.48, 147.31, 148.13, 151.47, 157.87, 162.12, 177.76.
Hydrolytic Stability Studies of CBS in Aqueous Acetone
The kinetics of hydrolysis of CBS was studied spectrophotometrically in acetone water systems using a thermostated Elico 198 Biospectrophotometer. The kinetic runs were carried out at different percentages of acetone – water system ranging from 20% to 60% (v/v) at 510 nm. All reactions were carried out under pseudo first order condition at 50°C. At room temperature the reaction is slow thus 50°C is selected for the hydrolysis studies. The observed first order rate constants were obtained from the slope of logarithmic concentrations of complex versus time graph.
Temperature effect on rate of hydrolysis was investigated by carrying out the hydrolysis reaction at five different temperatures ranging from 40 to 60°C. Arrhenius equation was used to calculate the activation energy and frequency factor for hydrolysis.23 To calculate thermodynamic activation parameters such as enthalpy of activation and entropy of activation, Eyring equation was used, 24
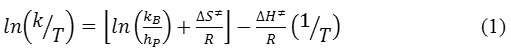
Eyring plot were drawn using ln(k/T) vs. 1/T and from slope and intercept of this straight line plot, ∆H# and ∆S# was calculated. Free energy of activation were calculated using the equation ∆G# = ∆H#-T∆S#.25
Data Analysis
Data analysis was performed using Microsoft Office Excel worksheet. The goodness of the fit was tested using the correlation coefficient and standard deviation.
Results and Discussion
Thermal Decomposition of CBS
Thermogravimetric analysis for CBS was carried out from room temperature to 700°C and the obtained TG-DTG curve is presented in Figure 2. The thermal decomposition of CBS proceeded with two distinct stages of decomposition. The first stage observed within the temperature range 125-180°C corresponds to the elimination of two molecules of crystal water attached to CBS with a mass loss of 7.05/7.00 % (calcd. /found). The second stage is observed at the temperature range 180-330°C, analogues to the loss of C6H5-CO-O group with a mass loss of 23.54/23.43 % (calcd. /found). These steps follow a continuous weight loss from 310-700°C due to the degradation of curcumin moiety. The pattern of decomposition is similar to that reported for bis-salicylatoborate of magnesium.26 The thermal degradation data is summarized in Table 1.
Table 1: Thermogravimetric data of CBS
|
Stages |
Temperature Range (°C) |
Ts (°C) |
Weight loss (%) |
Decomposed fragment |
|
|
Calculated |
Found |
||||
|
1 |
125-180 |
151 |
7.05 |
7.00 |
2 H2O |
|
2 |
180-330 |
256 |
23.54 |
23.43 |
C6H5-CO-O |
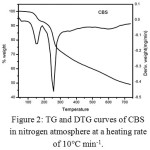 |
Figure 2: TG and DTG curves of CBS in nitrogen atmosphere at a heating rate of 10°C min-1. Click here to View figure |
Kinetic Aspects of Thermal Degradation
The kinetics of the thermal decomposition of CBS was studied by non-isoconversional method. The activation energy (Ea) and pre-exponential factor (A) were calculated using six methods given in Table 2 27-32. The kinetic parameters were determined from the slope and intercept of the straight line plot of each method mentioned in Table 2.
 |
Table 2: Different methods used for the kinetic analysis of thermal degradation of CBS. Click here to View table |
Linear plots with high correlation coefficient (0.9803-0.9982) were obtained for all methods which indicate that the decomposition is first order in two stages and there is a reasonable agreement between the experimental data and obtained kinetic parameters.33 The thermogravimetric parameters evaluated graphically using six different non-isothermal methods are listed in Table 3. The parameters calculated using all six methods are comparable and are in agreement with each other. For both decomposition steps, very close positive Ea values were observed, the second stage has higher Ea value than first decomposition step. The high Ea values indicate high thermal stability of CBS. The endothermic nature of thermal degradation was indicated by the positive value of enthalpy of activation energy. The positive values of entropy indicate the less ordered activated state and positive ΔG# values indicate the non-spontaneity of thermal degradation.
Table 3: Thermodynamic data of the thermal decomposition of CBS.
|
Stage |
Method |
R2 |
Ea (kJ mol-1) |
A (s-1) |
ΔH≠ (kJ mol-1) |
ΔS≠ (J K-1mol-1) |
∆G# (kJ mol-1) |
|
1 |
CR |
0.9951 |
173.30 |
5.13×1019 |
169.77 |
121.16 |
118.31 |
|
BR |
0.9955 |
178.75 |
1.52×1022 |
175.22 |
168.47 |
103.67 |
|
|
MKN |
0.9951 |
173.41 |
2.18×1022 |
169.00 |
169.64 |
97.04 |
|
|
SW |
0.9803 |
174.33 |
8.25×1017 |
169.93 |
84.99 |
124.88 |
|
|
HM |
0.9979 |
179.57 |
2.43×1020 |
174.84 |
131.65 |
99.93 |
|
|
FC |
0.9816 |
174.33 |
1.67×1017 |
169.42 |
70.83 |
127.64 |
|
|
2 |
CR |
0.9928 |
225.44 |
2.49×1020 |
221.04 |
132.46 |
150.83 |
|
BR |
0.9982 |
224.47 |
1.78×1021 |
220.06 |
148.83 |
141.18 |
|
|
MKN |
0.9974 |
215.91 |
1.53×1021 |
211.50 |
147.58 |
133.29 |
|
|
SW |
0.9817 |
214.71 |
2.29×1023 |
210.30 |
189.20 |
110.02 |
|
|
HM |
0.9979 |
226.27 |
3.23×1020 |
221.54 |
134.04 |
145.27 |
|
|
FC |
0.9896 |
214.71 |
1.35×1021 |
210.30 |
146.54 |
132.63 |
Hydrolytic Stability Studies of CBS
CBS stored in dry organic solvents like acetone, acetonitrile, DMSO, dioxane, etc. showed no change in its λmax value during the storage for three months in room temperature indicating its high solution state stability. Reduction in λmax value was observed during the addition of water. A systematic study was done in order to find the effect of water and temperature on the hydrolytic stability of CBS.
Effect of Water
The hydrolytic decomposition of CBS follows first order kinetics with complete decomposition as shown by zero absorbance for infinity absorbance measurement for every kinetic run. Hydrolysis of CBS was carried out at 50°C in different percentages of acetone-water mixtures. The first order rate constants obtained are summarized in Table 4. The results show that the rate constant k1 increases with increase in the water percentage of reaction medium. The mole fraction versus rate constant plot is shown in Figure 3. A linear plot with a correlation coefficient (R2) 0.9984 with a negative slop indicate the rate of reaction is directly proportional to the concentration of water in the reaction medium and the reaction proceed by a simple mechanistic path.
Table 4: First order rate constant for the hydrolysis of CBS in different % of acetone – water medium at 50°C.
|
V (%) acetone |
Mole fraction of acetone |
Rate constant 104 k1 (s-1) |
|
20 |
0.057 |
9.30±0.03 |
|
30 |
0.095 |
7.25±0.04 |
|
40 |
0.140 |
5.45±0.01 |
|
50 |
0.196 |
3.95±0.03 |
|
60 |
0.268 |
3.23±0.02 |
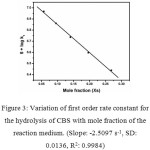 |
Figure 3: Variation of first order rate constant for the hydrolysis of CBS with mole fraction of the reaction medium. (Slope: -2.5097 s-1, SD: 0.0136, R2: 0.9984) Click here to View figure |
The Grunwald-Winstein plot were drawn using log(k/ko) against solvent ionizing power scale, Y as shown in Figure 434-35. The m value obtained from the slope of the GW plot for the hydrolysis of CBS is 0.3871, which indicates that the intermediate formed during the hydrolysis is chargeless36.
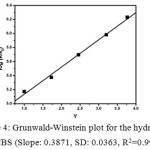 |
Figure 4: Grunwald-Winstein plot for the hydrolysis of CBS (Slope: 0.3871, SD: 0.0363, R2=0.9973) Click here to View figure |
Effect of Temperature
The influence of the temperature on the hydrolysis rate constant of CBS was determined over the temperature range 40 to 60 oC in 50% (v/v) acetone water system and the kinetic parameters at standard temperature 298 K are presented in Table 5 along with the measured first order rate constants. Arrhenius plot showed a linear relationship between ln k and reciprocal of absolute temperature with correlation coefficient 0.9972 and standard deviation (SD) 0.0653 as shown in Figure 5a. The Eyring plot (Figure 5b) also showed a linear relationship with standard deviation 0.0420 and correlation 0.9988.
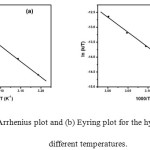 |
Figure 5a: Arrhenius plot and (b) Eyring plot for the hydrolysis of CBS at different temperatures.
|
Table 5: Kinetic and thermodynamic parameters for the hydrolysis of CBS in 50 % acetone water system.
|
T (°C) |
104 k (s-1) |
k at 25oC (s-1) |
Ea (kJ mol-1) |
A (s-1) |
ΔH≠ (kJ mol-1) |
ΔS≠ (J K-1mol-1) |
∆G# (kJ mol-1) |
|
40 |
1.42 |
2.67×105 |
86.33 |
3.21×1037 |
83.64 |
-51.88 |
99.11 |
|
45 |
2.44 |
||||||
|
50 |
3.85 |
||||||
|
55 |
6.14 |
||||||
|
60 |
10.8 |
The higher value of enthalpy of transition state indicates that the energy requirement for the formation of intermediate during the hydrolysis of CBS is high. Negative value of ΔS# indicates that activated complex is more ordered than the reactant and no significant change occurs in the internal structure of CBS during the formation of activated complex. The ∆G# value is positive which indicates the hydrolysis reaction as a thermodynamically feasible reaction. Thus the kinetic parameters indicate the possibility of a bulky transition state during hydrolysis36.
Mechanism for the Hydrolysis of CBS
UV-Visible, 11B NMR spectral studies and HPLC analysis were used to study the product formed during the hydrolysis of CBS. The UV-visible spectra of CBS recorded during the hydrolysis shows a decreasing peak at 510 and an increasing peak at 420 nm. The peak at 420 is due to the formation of curcumin as one of the hydrolysis product.37 Figure 6 is the 11B NMR spectrum of CBS recorded at room temperature, three hours after the hydrolysis has begun. The spectrum shows that solution contains two different boron species. The peak at 6.73 corresponds to tetrahedral boron atom present in CBS. The trigonal boron atom present in boric acid gives a signal at δ ~ 19 38 which is not observed in this case. An additional signal observed at δ 22.99 can be ascribed to tetrahedral boron [B(OH)–4], as one of the hydrolysis product. The product analysis by HPLC in methanol medium confirms the presence of curcumin and salicylic acid as hydrolysis product.
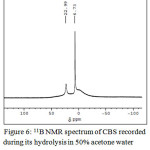 |
Figure 6: 11B NMR spectrum of CBS recorded during its hydrolysis in 50% acetone water system at room temperature.
|
The formation of bis-chelate boron salicylic acid complex was reported to takes place by a two-step mechanism, where the mono-chelate complex was formed in the first stage followed by the condensation reaction with fully protonated salicylic acid to form bis-chelate complex.39 The condensation takes place by the transfer of one of the hydrogen from the ligand to hydroxyl group of boric acid to eliminate as a water molecule.The significance of proton transfer for the complexation reaction of boric acid is demonstrated by the early work of Pizer and co-workers.40-41 For the reverse process, the hydrolysis of bis-chelate, the addition of water molecule to liberate the fully protonated salicylic acid and mono-chelate boron complex can be suggested followed by the rapid hydrolysis monochelate.
In the hydrolysis reaction the oxygen atom of the entering water molecule interact the central boron as nucleophile, simultaneously the hydrogen form an intermolecular hydrogen bond with the B-O-C oxygen to form a cyclic transition state as shown in Scheme 2. The nucleophilic attack of –OH group towards boron atom to form a five coordinated transition state in rate determining step has been reported in the hydrolysis of four coordinate boron complexes.42-43 The interaction of water molecule to boron was reported in the complexation of boric acid with catechols and salicylic acid.26,44 Shao and co-workers have postulated the formation of five co-ordinated boron intermediate during the hydrolysis of boric acid complex with chromotropic acid.44
The present data is not sufficient to predict the exact nature of the complex formed during hydrolysis. However it can be shown that the B-O-C bond and the O-H bond of hydrogen bonded water molecules breaks resulting in the hydrolysis of complex, the further addition of water resulted in the removal of salicylate moiety from the complex. The extended conjugation of curcumin with boron species suggest the removal of salicylate moiety followed by curcumin from the complex. Since the complex possess a highly symmetric structure and the oxygen atom present in µ-oxo bridge possess very low electron density the addition of hydrogen atom to the complex is a very slow process. Therefore the rate determining step is the formation of bulky intermediate through hydrogen bonding.44 The values of thermodynamic parameters also support the formation of moderately stable complex. The positive value of ∆H# favors the formation of complex and the negative ∆S# value indicates the formation of a rigid structure.
CBS has four B-O bonds that are vulnerable to hydrolytic attack. The hydrolysis of CBS begins with the cleavage of two B-O bonds between salicylic acid and boron through the formation hydrogen bond with water which results in the release of salicylic acid along with tetrahedral curcumin boron complex similar to that of boron complex of chromotropic acid.44 11B NMR spectrum confirms the absence of trigonal boron in hydrolysis process. The absence of any specific peak in HPLC analysis suggest a very fast second stage process resulting in the formation of curcumin and [B(OH)4– ].
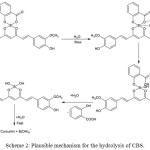 |
Scheme 2: Plausible mechanism for the hydrolysis of CBS. |
Conclusion
Spiroborate ester of curcumin with salicylic acid has been synthesized and characterized. Thermal degradation behavior was analyzed by TG-DTG technique and kinetic parameters associated with thermal degradation were evaluated using different non-isothermal methods. The values obtained from these methods are comparable and are in good agreement with each other. Hydrolytic stability of the complex was studied spectrophotometrically in acetone water system at different percentage of water and temperature. The reaction followed first order kinetics and hydrolysis rate increased with increase in temperature and percentage of water. Kinetic data obtained from the hydrolysis study indicates the possibility of a bulky intermediate during hydrolysis. UV-Visible, HPLC and 11B NMR spectral analysis were conducted to analyze the hydrolysis product. Based on that a plausible mechanism for the hydrolysis was suggested.
Acknowledgement
The financial support from the Council of Scientific and Industrial Research (Grant No. 08/538/(0003)/2012-EMR-1), New Delhi is gratefully acknowledged. Authors would also like to thank SAIF, Cochin and IISER Thiruvananthapuram for carrying out spectral analysis.
References
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R. K. Turmeric and curcumin: Biological actions and medicinal applications. Current Science, 2004, 87, 44-53.
- Gupta, A.P.; Gupta, M.M.; Kumar, S. Simultaneous determination of curcuminoids in curcuma samples using high performance thin layer chromatography. J. Liq. Chrom. & Rel. Technol. 1999, 22, 1561-69.
- Soudamini, K.K.; Kuttan, R. Inhibition of chemical carcinogenesis by curcumin. J. Ethnopharmacol. 1989, 27, 227-233.
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem.2006, 98, 720-724.
- Khopde, S.M.; Priyadarsini, K.I.; Venkadesan, U.P.; Rao, M.N.A. Free radical scavenging ability and antioxidant efficiency of curcumin and its substituted analogue. Biophys. Chem. 1999, 80, 85-91.
- Elizabeth, K; Rao, M.N.A. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237-240.
- Nurfina, A.N.; Reksohadiprodjo, M.S.; Timmerman, H.; Jenie, U.A.; Sugiyanto, D.; van der Goot, H. Synthesis of some symmetrical curcumin analogues and their anti-inflammatory activity. Eur. J. Med. Chem. 1997, 32, 321-28.
- Mazumder, A.; Neamabi, N.; Sunder, S.; Schilz, J.; Pertz, H.; Eich, E.; Pommier, Y. Curcumin analogs with altered potencies against HIV-1 integrase as probes for biochemical mechanisms of drug action. J. Med. Chem. 1997, 40, 3057-3063.
- Akram, M.; Shahab-Uddin; Ahmed, A.; Usmangghani, K.; Hannan, A.; Mohiuddin, E.; Asif, M. Curcuma longa and curcumin: A review article. J. Biol-Plant Biol. 2010, 55, 65-70.
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial antiviral and antifungal activity of curcumin. Biomed. Res. Int. 2014, 2014, 186864-76.
- Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioinorg. Med. Chem. 2009, 17, 2623-2631.
- Nugroho, A.E.; Ikawati, Z.; Sardjiman; and Maeyama, K. Effects of benzylidene- cyclopentanone analogues of curcumin on histamine release from mast cells. Biol. Pharm. Bull. 2009, 32, 842-849.
- Changtam, C.; Koning, H.P.; Ibrahim, H.; Sajid, M.S.; Gould, M.K.; Suksamrarn, A. Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species Eur. J. Med. Chem. 2010, 45, 941-956
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N.P. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives Biol. Pharm. Bull. 2007, 30, 74-78.
- Uppstrom, L.R.; Ostling, G. A modified method for determination of boron with curcumin and a simplified water elimination procedure. Anal. Lett. 1976, 9, 311–324.
- Dyrssen, D.W.; Uppstrom, L.R.; Novikov, Y.P. Studies on the chemistry of the determination of boron with curcumin. Anal. Chim. Acta, 1972, 60, 139-151.
- Sui, Z.; Salto, R.; Li, J.; Craik, C.; Ortiz de Montellano, P.R. Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg. Med. Chem. 1993, 1, 415-422.
- Ran, C.; Xu, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, synthesis and testing of difluoroboron-derivatized curcumins as near-infrared probes for in-vivo detection of amylode-beta deposits, J. Am. Chem. Soc. 2009, 131, 15257− 15261
- Randjelovic, P.; Veljkovic, S.; Stojilijkovic, N.; Sokolovic, D.; Ilic, I.; Laketic, D.; Randjelovic, D.; Randjelovic, N. The beneficial biological properties of salicylic acid, Acta facultatis medicae Naissensis. 2015, 32, 259-265.
- Goncalves, G.M.S.; da Silva, G.H.; Barros, P.P.; Srebernich, S.M.; Shiraishi, C.T.C.; de Camargos, V.R.; Lasca, T.B. Use of Curcuma longa in cosmetics: extraction of curcuminoid pigments, development of formulations, and in vitro skin permeation studies. Braz. J. Pharm. Sci., 2014, 50, 885-893.
- See, A.S.; Salleh, A.B.; Bakar, F.A.; Yusof, N.A.; Abdulamir A.S.; Heng, L.Y. Risk and health effect of boric acid, Am. J. Appl. Sci. 2010, 7, 620-627.
- Asha, R.; Devi, R.S.; Priya, R.S.; Balachandran, S.; Mohanan, P.V.; Abraham, A. Bioactive derivatives of curcumin attenuate cataract formation in vitro. Chem. Biol. Drug Des. 2012, 80, 887.
- Svirbely, W.J.; Kundell, F.A. The kinetics of competitive-consecutive second-order reactions involving difunctional unsymmetrical molecules. The kinetics of the alkaline hydrolysis of diethyl malate J. Am. Chem. Soc. 1967, 89, 5354.
- Al-Ghouti, M.; Khraishehb, M.A.M.; Ahmada, M.N.M.; Allen, S. Thermodynamic behavior and the effect of temperature on the removal of dyes from aqueous solution using modified diatomite: A kinetic study. J. Colloid Interface Sci. 2005, 287, 6.
- Sismanoglu, T.; Pura, S. Adsorption of aqueous nitrophenols on clinoptilolite. Colloids Surf A: Physicochem. Eng. Asp. 2001, 180, 1.
- Kose, D.A.; Zumreoglu-Karan, B.; Hokelek, T. A comparative examination of mono- and bis-chelate salicylatoborate complexes and the crystal structure of layered magnesium bis-salicylatoborate, Inorg. Chim. Acta. 2011, 375, 236-241.
- Coats, A.W.; Redfern, J. P. Kinetic parameters from thermogravimetric data. Nature, 1964, 201, 67.
- Broido, A.A. A simple, sensitive graphical method of treating thermogravimetric analysis data. J. Polym. Sci. 1969, 2A, 1761.
- Madhusudhanan, P.M.; Krishnan, K.; Ninan, K. N. New approximation for the p(x) functions in the evaluation of non-isothermal kinetic data. Thermochim. Acta, 1986, 97, 189.
- Sharp, J. B.; Wentworth, S.A. Kinetic analysis of thermogravimetric data. Anal. Chem. 1969, 41, 2060.
- Horowitz, H.H.; Metzger, G. A new analysis of thermogravimetric traces. Anal. Chem. 1963, 35, 1465.
- Freeman, E.S.; Carroll, B. The application of thermoanalytical techniques to reaction kinetics: The thermogravimetric evalution of the kinetics of the decomposition of calcium oxalate monohydrate. J. Phys. Chem. 1958, 62, 394.
- Adly, O.M.I.; Taha, A.; Fahmy, S.A. Synthesis, spectral characterization, molecular modeling and antimicrobial activity of new potentially N2O2 schiff base complexes, J. Mol. Struct. 2013, 1054, 239-250.
- Winstein, S.; Grunwald, E.; Jones, H.W. The correlation of solvolysis rates and the classification of solvolysis reactions into mechanistic categories. J. Am. Chem. Soc. 1951, 73, 2700.
- Grunwald, E.; Winstein, S. The Correlation of solvolysis rates. J. Am. Chem. Soc. 1948, 70, 846.
- Koh, H.J.; Kang, S.J. Application of the extended Grunwald-Winstein equation to the solvolysis of phenyl methane sulfonyl chloride in aqueous binary mixtures. Bull. Korean Chem. Soc. 2011, 32, 1897.
- Chignell, C.F.; Bilski, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral and photochemical properties of curcumin, Photochem. Photobiol. 1994, 59, 295-302.
- Kose, D.A.; Karan, B.Z. Mixed-ligand complexes of boric acid with organic biomolecules, Chemical papers, 2012, 66, 54-60.
- Basset, J.; Mathews, P.J. The preparation and properties of some bis(salicylato) borate (III) salts with large cations, J. Inorg. Nucl.Chem. 1978, 40, 987-992.
- Pizer, R.; Babcock, L. Mechanism of the complexation of boron acids with catechol and substituted catechols, Inorg. Chem. 1977, 16, 1677-1681
- Friedman, S.; Pizer, R. mechanism of the complexation of phenyl boronic acid with oxalic acid. Reaction which requires ligand donor atom protonation. J. Am. Chem. Soc. 1975, 97, 6059-6062.
- Pepperberg, I.M.; Halgren, T.A.; Lipscomb, W.N. A molecular orbital study of the role of BH5 in the hydrolysis of BH4– . J. Am. Chem. Soc. 1976, 98, 3443.
- Kreevoy, M.M.; Hutchins, J.E.C. H2BH3 as an intermediate in tetrahydridoborate hydrolysis. J. Am. Chem. Soc. 1972, 94, 6371.
- Shao, C.; Matsuoka, S.; Miyazaki, Y.; Yoshimura, K.; Suzuki, T.M.; Tanaka, D.A.P. Equilibrium and kinetic studies on the complexation of boric acid with chromotropic acid. J. Chem. Soc. Dalton Trans. 2000, 18, 3136.

This work is licensed under a Creative Commons Attribution 4.0 International License.









