Adsorption Study of Pb(II), Cd(II), Cu(II) and Cr(III) Ions in Aqueous Medium using C-4-Hydroxy-3 Methoxyphenylcalix[4]Resorcinarene Dodecaacetate
Chairil Anwar, Jumina, Dwi Siswanta, Sri Juari Santosa, Agnisa Fiani and Muhammad Idham Darussalam Mardjan
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, Sekip Utara Yogyakarta 55281.
Corresponding Author E-mail: chanwar@ugm.ac.id
DOI : http://dx.doi.org/10.13005/ojc/330251
Article Received on : February 13, 2017
Article Accepted on : March 14, 2017
This research work presented the application of C-4-hydroxy-3-methoxyphenylcalix[4]resorcinarene dodecaacetate in the removal of Pb(II), Cd(II), Cu(II) and Cr(III) in aqueous medium. Various adsorption parameter such as pH, adsorbent dose and interaction time were investigated. In addition, the kinetic study was conducted by using Santosa's first order, Lagergren's pseudo-first order and Ho's pseudo-second order models. The results showed that the optimum pH for the sorption of Pb(II) and Cu(II) was 4 and that for Cd(II) and Cr(III) was 5. The optimum adsorbent dosage was 0.0175 g for all the metal ions. While the adsorption kinetic for Cd(II), Cu(II) and Cr(III) was relatively fast, the adsorption of Pb(II) occurred in slower rate. Therefore, the kinetic study be solely performed for the adsorption of Pb(II). The adsorption of Pb(II) followed the pseudo-second order of Ho with the equilibrium adsorption capacity and rate constant of 0.3936 mg/g and 0.0865 g/mg.min, respectively. From this study, C-4-hydroxy-3-methoxyphenylcalix[4]resorcinarene dodecaacetate was expected to become an alternative adsorbent in the removal of heavy metals.
KEYWORDS:C-4-hydroxy-3-methoxyphenylcalix[4]resorcinarene dodecaacetate; adsorption; heavy metals
Download this article as:| Copy the following to cite this article: Anwar C, Jumina J, Siswanta D, Santosa S. J, Fiani A, Mardjan M. I. D. Adsorption Study of Pb(II), Cd(II), Cu(II) and Cr(III) Ions in Aqueous Medium using C-4-Hydroxy-3-Methoxyphenylcalix[4]Resorcinarene Dodecaacetate. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Anwar C, Jumina J, Siswanta D, Santosa S. J, Fiani A, Mardjan M. I. D. Adsorption Study of Pb(II), Cd(II), Cu(II) and Cr(III) Ions in Aqueous Medium using C-4-Hydroxy-3-Methoxyphenylcalix[4]Resorcinarene Dodecaacetate. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=32354 |
Introduction
Heavy metal contamination in water is a serious problem, particularly in the health and environmental aspects. It occurs since many industries discharge their waste without any proper treatment. Most of the heavy metal are toxic and may be bio-accumulated in human body. In addition, heavy metal may cause serious health hazards1. In this context, WHO regulated the standard quality of metal ion in the drinking water. For instance, the regular limits for Pb(II), Cd(II), Cu(II) and Cr(III) should not exceed 0.01, 0.003, 2.0 and 0.05 mg/L, respectively2. To eliminate the amount of metal ions in the aquatic environment, a proper and effective wastewater treatment is highly required. Various techniques have been developed such as adsorption, ion-exchange, coagulation and precipitation. Among them, adsorption offers several advantageous such as simple operation, good efficiency and the possibility to recycle the adsorbent. It is also cheap and can be applied at low concentration of metal ions3. A number of natural and synthetic adsorbents have been applied in the removal of heavy metal ions such as zeolite4, humic acid5, modified chitosan6-8, biosorbent9 and calixarene10-11. Calixarene is a cyclic oligomer of phenol which posses unique structure and shape. The presence of cavity and the unlimited functional group modification has led this macrocycle to be mainly applied in host-guest chemistry12. This study will more focus on the cyclic oligomer of resorcinol namely calix[4]resorcinarene. The previous reports showed that calix[4]resorcinarene was significantly efficient in heavy metal adsorption13-17. The design of calix[4]resorcinarene-based-adsorbent was carried out by installing numbers of chelating atoms into its framework. The potential functional groups which display good affinity towards the metal ions include amine, alcohol, ether, phosphine, carbonyl and carboxylic acid derivatives8, 18-21. In the connection with our study, we have synthesized C-4-hydroxy-3-methoxyphenylcalix[4]resorcinarene dodecaacetate containing acetoxy and alkoxy moieties. Beside the simple adsorbent preparation, it was expected that the increase of chelating atoms may be beneficial to the heavy metal sorption. Therefore, the adsorption performance of the synthesized calix[4]resorcinarene in the removal Pb(II), Cd(II), Cu(II) and Cr(III) ions will be investigated.
Experimental Part
Materials and Instrumentation
Adsorbent of C-4-hydroxy-3-methoxyphenylcalix[4]resorcinarene dodecaacetate was prepared according to the previous study (Figure 1). The adsorbent was sieved to give the particle size of 100 mesh. The stock solutions of Pb(NO3)2, Cu(NO3)2, Cd(NO3)2 and Cr(NO3)3 (1,000 mg/L in HNO3 0.5 M) were purchased from E. Merck. The stock solution was diluted with deionized water to give the desired concentration. The concentration of the metal ions was determined by atomic absorption spectrometer (AAS, Perkin Elmer 3110 USA) and was corrected with the blank solution. Solutions of HCl (0.1 M) and NaOH (0.1 M) were utilized to adjust the pH of the solution. The pH was determined by pH meter (Orion model 370).
![Figure 1: The Structure of C-4-hydroxy-3-methoxyphenylcalix [4]resorcinarene dodecaacetate](http://www.orientjchem.org/wp-content/uploads/2017/04/Vol33No2_Adso_Chai_fig1-150x150.jpg) |
Figure 1: The Structure of C-4-hydroxy-3-methoxyphenylcalix [4]resorcinarene dodecaacetate
|
Adsorption Study
The adsorption was carried out in batch system. As much as 0.1 g of the adsorbent was introduced into the 10 mL of metal solution in certain concentration and followed with the shaking at temperature ambient for certain period of time. The filtration of the mixture gave the filtrate which was analyzed by means of AAS spectrometer to determine the metal concentration. Additionally, the concentration of metals in blank solution was also measured. The adsorption capacity q (mmol/g) for such experiment was calculate based on Eq. 1.
![]()
where Co was the initial concentration of metal ion, Cf was the metal ion concentration after certain period of time. The volume of metal solution and the mass of the adsorbent were defined as V and W, respectively.
In this study, several adsorption parameters were evaluated including pH, mass of adsorbent and interaction time. The kinetic study was also performed by using three models. The first-order model was proposed by Santosa22 which was based on the concentration of the adsorbate in the aqueous phase and can be described by the eq. 2.
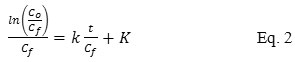
where t was adsorption time (minute), k was first order adsorption rate constant (minute-1) and K was equilibrium constant (mol/L). In addition, the next two models (Lagergren and Ho) were based on the concentration of the adsorbate in the solid phase. The Lagergen’s pseudo-first order and the Ho’s pseudo-second order were generally expressed by eq. 3 and 423-24, respectively.

where qe and qt were the adsorption capacity (mg/g) at the equilibrium and contact time t, while k1 and k2 were the rate constants of the pseudo-first order (mg.g-1.min-1) and the pseudo-second order (g.mg-1.min-1), respectively.
Results and Discussions
We have performed adsorption study of some selected heavy metal cations (Pb(II), Cd(II), Cu(II) and Cr(III)) by using C-4-hydroxy-3-methoxyphenylcalix[4]resorcinarene dodecaacetate as adsorbent. The study was carried out under different pH, adsorption dose and contact time in order to determine the maximum adsorption capacity. In addition, the kinetic study was also conducted to determine the kinetic mechanism of the adsorption of the heavy metals.
Effect of pH
pH is one of the important parameter in the adsorption study since it may affect the nature of both adsorbent surface and the metal ion25. In this study, the adsorption was carried out in the range pH of 2-6. As displayed on Figure 2, the adsorption profiles for all metal ions were dependant to the pH value and displayed similar pattern. In acidic condition, the adsorption capacity was relatively low. Increasing the pH enhanced the capacity until the optimum pH and the higher pH led to the decrease of adsorption capacity.
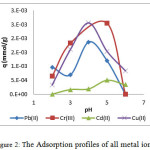 |
Figure 2: The Adsorption profiles of all metal ions
|
These results can be explained by considering electronic interaction between the adsorbent and the speciation of metal ions26-28 in different pH. In term of metal speciation, both Pb and Cd are mainly existed as Pb2+ and Cd2+ under pH 6. On the other hand, the major species of Cr3+ and Cu2+ can be found under pH 2 and 4, respectively. Increasing the pH value allowed to these metal ion to coordinate with ligand exists on the system to form the metal complex which posses lower positive charge (M(n-x)+).
In low pH, all the metal ions were in the form of free metal ion (Mn+). However, the proton was also present in the medium and was the potential Lewis acid to interact with the adsorbent. Therefore, the competition between the proton and the metal ion may decrease the adsorption capacity. The increase of adsorption capacity toward all the metal ions were observed starting from pH 3 until the optimum pH values were reached. The rationalization of these results was probably due to the decrease of the proton concentration. Therefore, the adsorbent will preferably interact with the metal ion. While the optimum pH of Pb(II) and Cu(II) was 4, the maximum adsorption capacity for Cd(II) and Cr(III) was reached at pH 5. Performing the study above the optimum pH diminished the adsorption capacity due to the increasing of the hydroxyl ion concentration. This allowed the formation of lower charge metal complex and, therefore, decrease the interaction between metal and adsorbent.
Effect of Adsorbent Dose
The effect of the adsorption dosage on the removal of the metal ions was investigated by varying the adsorbent mass ranged from 0.0175 to 0.15 g (Figure 3). The study was carried out at the initial metal concentration of 10 ppm at temperature ambient and under the optimum pH. It was expected that increasing the dose may supply more the adsorption sites and may, therefore, enhance the capacity. However, we observed the decrease on the adsorption capacity for all the metal ions with the increasing of the adsorption dose. The reason may be owing to the aggregation of the adsorbent due to the high concentration of adsorbent. As the consequence, the available adsorption sites were decreased29.
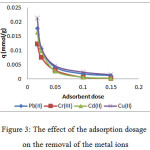 |
Figure 3: The effect of the adsorption dosage on the removal of the metal ions
|
Effect of Contact Time
The effect of contact time to the removal of metal ion using calix[4]resorcinarene adsorbent was presented in Figure 4 and 5. The adsorption of Cu(II), Cd(II) and Cr(III) dramatically increased at 3 min. After 3 min of interaction time, the adsorption capacity of Cd(II) was relatively constant, while that of Cu(II) and Cr(III) tended to decrease. The decrease on the capacity was probably due to the weak interaction between the adsorbent and the metal ion. On the other hand, the rate of Pb(II) adsorption was relatively low comparing to the others. The rapid increase of the adsorption capacity occurred at the first 30 min. The capacity gradually increased and reached the equilibrium at 360 min.
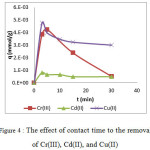 |
Figure 4 : The effect of contact time to the removal of Cr(III), Cd(II), and Cu(II) |
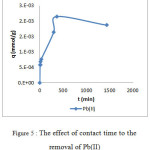 |
Figure 5: The effect of contact time to the removal of Pb(II)
|
Kinetic Study
In order to investigate the mechanism of metal sorption on calix[4]resorcinarene adsorbent, three kinetic equation models were utilized. They were first order of Santosa, pseudo first-order of Lagergren and pseudo-second order of Ho. Unfortunately, the adsorption kinetic of Cu(II), Cd(II) and Cr(III) ions could not be studied due to the very rapid sorption at the initial stage and the decrease of capacity as the function of time.
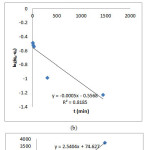 |
Figure 6 : The three kinetic models (a) Santosa’s first order (b) Lagergren’s pseudo-first order (c) Ho’s pseudo-second order
|
Table 1: The three kinetic profiles of the adsorption of Pb(II) onto the calix[4]resorcinarene
|
Kinetic Parameter |
Pb(II) |
|
| qe (mg/g) |
0.4454 |
|
| Santosa’s first order | k (1/min) |
0.0004 |
| r2 |
0.8823 |
|
| Lagergren’s pseudo-first order | k1 (1/min) |
0.0012 |
| qe (mg/g) |
0.2775 |
|
| r2 |
0.8185 |
|
| Ho’s pseudo-second order | k2 (g/mg.min) |
0.0865 |
| qe (mg/g) |
0.3936 |
|
| r2 |
0.9995 |
|
The kinetic profiles of the adsorption of Pb(II) onto the calix[4]resorcinarene by using the three kinetic models were depicted in (Figure 6a-c and Table 1). By comparing the value of the linear regression value (R2) of each model, it was found that Ho’s pseudo-second order (R2 = 0.9995) had the highest linearity comparing to Santosa’s first order (R2 = 0.8823) and Lagergren’s pseudo-first order (R2 = 0.8185). It indicated that that adsorption process could be better described by the second order of Ho.
According to the Ho’s equation, the adsorption capacity at the equilibrium can be determined from the slope of the plot of t/qt vs t. The capacity was 0.3936 mg/g and was more closer to the experimental adsorption capacity. Moreover, the calculated rate constant (k2) was found to be 0.0865 g/mg.min.
Conclusion
Adsorption study of several heavy metal ions of Pb(II), Cd(II), Cu(II) and Cr(III) onto C-4-hydroxy-3-methoxyphenylcalix[4]resorcinarene dodecaacetate has been successfully carried out. The results showed that the adsorbent was effective to remove Pb(II) and Cu(II) at pH 4 as well as Cd(II) and Cr(III) at pH 5. It was noticed that the increase of the adsorbent dose led to the decrease of adsorption capacity. It has been also found that the adsorption rate for Pb(II) was relatively lower than the that for the others. Moreover, the adsorption of Pb(II) followed the pseudo second order model of Ho. From this study, C-4-hydroxy-3-methoxyphenylcalix[4]resorcinarene dodecaacetate was expected to become an alternative adsorbent in the removal of heavy metals.
References
- Bailey, S.E.; Trudy, J.; Olin, T.J.; Bricka, M.R.; Adrian,D.D. Water.Res. 1999, 33 (11), 2469–2479.
CrossRef - W.H.O. Guidelines for drinking-water quality, 4th Ed., Geneva, WHO Press 2011.. 327, 340, 383.
- Ren, Y.; Zhang, M.; Zhao, D. Desalination 2008, 228, 135-149.
CrossRef - Blanchard, G.M. ; Maunaye ; Martin, G. Water.Res. 1984, 18 (12), 1501-1507.
CrossRef - Jin, X. ; Bailey, G.W. ; Yu, Y.S., Lynch, A.T. Soil Science 1996, 161 (8), 509-520.
CrossRef - Tabakci, M.; Yilmaz, M. Bioresour. Technol. 2008, 99, 6642-6645.
CrossRef - Li, H-B.; Chen, Y-Y.; Liu, S-L. J. Appl. Polym. Sci. 2003, 89, 1139-1144.
CrossRef - Siswanta, D.; Jumina ; Anggraini, M; Mardjan, M.I.D.; Mulyono, P.; Ohto, K. Int. J. Appl. Chem. 2016, 12 (1), 11-22.
- Dang, V.B.H.;Doan,H.D.; Dang-Vuc, T.; Lohi, A. Bioresour. Technol. 2009, 100, 211-219.
CrossRef - Tabakci,M.; Yilmaz,M. J. Hazard Mater. 2008, 151, 331-338.
CrossRef - Tabakci, M.; Erdemir, S.; Yilmaz, M. J. Hazard Mater. 2007, 148, 428-435.
CrossRef - Gutsche,C.D. Calixarenes, Monograph in Supramolecular Chemistry, Cambride, Royal Society of Chemistry. 1989.
- Al-Trawneh, S.A. Jordan J. Earth Env. Sci. 2015, 7 (1), 1-9.
- Utomo, S.B.; Jumina; Siswanta, D.; Mustofa. Indones. J. Chem. 2012., 12 (1), 49-56.
CrossRef - Jumina; Sarjono, R.E.; Paramita, B.W.; Siswanta, D.; Santosa, S.J.; Anwar, C.; Sastrohamidjojo, H.; Ohto, K.; Oshima, T. J. Chin. Chem. Soc. 2007, 54 (5), 1167-1178.
- Jumina; Sarjono, R.E.; Siswanta, D.; Santosa, S.J.; Ohto, K. J. Korean Chem. Soc. 2011, 55 (3). 454-462.
- Kesuma, F.E.; Jumina; Ohto, K.; Siswanta, D. Orient. J. Chem. 2016, 32 (2), 769-775.
CrossRef - Handayani, D.S.; Jumina; Siswanta, D.; Mustofa; Ohto, K.; Kawakita, H. Indones. J. Chem. 2011, 11 (2), 191-195.
- Adhikari, B.B.; Kanemitru, M.; Kawakita, H.; Jumina; Ohto, K. Chem. J. Eng. 2011, 172, 341-353.
CrossRef - Adhikari, B.B.; Ohto, K.; Schramm, M.P. Chem. Commun. 2014, 50, 1903-1905.
CrossRef - Adhikari, B.B.; Gurung, M.; Chetry, A.B.; Kawakita, H.; Ohto, K. RSC. Adv. 2013, 3, 25950-25959.
CrossRef - Santosa, S.J.; Siswanta, D.; Sudiono. S.; Sehol, M. Surf. Sci. 2007, 601, 5148-5154.
CrossRef - Ho, Y.S.; McKay, G. Process Biochemistry 1999, 34, 451-465.
CrossRef - Ho, Y.S. J. Hazard. Mater. 2006, 136, 681-689.
CrossRef - Wu, Y.H.; Jiang, L.; Mi, X.M.; Li, B.; Feng, S.X. Korean J. Chem. Eng. 2011, 28 (3), 895-901.
CrossRef - Berber-Mendoza, M.S.; Leyva-Ramos, R.; Alonso-Davila, P.; Mendoza-Baron, J.; Diaz-Flores, P.E. J. Chem. Tech. Biotech. 2006, 81, 966-973.
CrossRef - Odisitse, S.; Jackson, G.E.; Govender, T.; Kruger, H.G.; Singh, A. Dalton Trans. 2007, 1140-1149.
CrossRef - Santos, V.C.G.D.; Salvado, A.D.P.A.; Dragunski, D.C.; Peraro, D.N.C.; Tarley, C.R.T.; Caetano, J. Quim. Nova. 2012, 35 (8), 1606-1611.
CrossRef - Ramesh, A.; Hasegawa, H.; Maki, T.; Ueda, K. Sep. Purif. Technol. 2007, 56 (1), 90-100.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









