Synthesis, Characterisations, Lipid Lowering Activity and Antibacterial Activities of Some Benzoyl Ester Substituted Embelin Derivatives
Ashok N. Patange
Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), University of Mumbai, Mumbai 400001, Maharashtra 400058, India.
Corresponding Author E-mail: ashokpatange78@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330150
The research article represents the extraction of Embelin from its plant source and synthesis, characterisation, lipid lowering activities and antibacterial studies of some Benzoyl ester substituted derivatives of Embelin. The derivatives of Embelin , EL-1 to EL-5 were prepared by treating Embelin with Meta and Para substituted benzoyl chloride in presence of base pyridine and dichloromethane. Benzoyl ester substituted derivatives of Embelin molecule also shows more or less medicinal properties then the parent Embelin molecule.The synthesized compound were characterised and identified on the basis of elemental analysis, UV, FTIR, Mass and NMR spectroscopy and their antibacterial activities were studied against test organism viz B.subtilis gram positive,S.Aureus gram positive , C.Albicans Antifungal and E.Coli gram negative. The lipid lowering activities of prepared compound were also studied in percentage on pancreatic lipase inhibition simply on the basis of change in international unit (IU).
KEYWORDS:embelia ribes; IC50 value; B.subtilis gram positive; S.Aureus gram positive; C.Albicans antifungal and E.Coli gram negative
Download this article as:| Copy the following to cite this article: Patange A. N. Synthesis, Characterisations, Lipid Lowering Activity and Antibacterial Activities of Some Benzoyl Ester Substituted Embelin Derivatives. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Patange A. N. Synthesis, Characterisations, Lipid Lowering Activity and Antibacterial Activities of Some Benzoyl Ester Substituted Embelin Derivatives. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=30579 |
Introduction
It has been long recognised that natural products acts as a lead structures for various drug discovery 1-2 because of their high chemical diversity, biochemical specificity and other molecular properties that make them favourable as a drug which serve to differentiate them from libraries of synthetic and combinatorial compounds 3 .In the traditional Indian medicine Embelia ribes is used as medicinal plant for the treatment of various ailments. Embelin (3-undecyl 2,5- dihydroxy ,1,4 –benzoquinone) was an important naturally occurring alkyl substituted hydroxyl benzoquinone product and one of the main constituent of embelia ribes burm ,fruits of the plants contain a quinine derivative embelin an alkaloid chiristembine 4 and volatile oil vilangin ,its chemical constituent is 2,5- dihydroxy -4-undecyl- 3,6 –benzoquinone5.the variety of biological activities of this compounds have been studied and evaluated for anti spermatogenic effect 6 and urinary tract infection 7.literature review shows that the phytochemical and pharmacological properties of embelia ribes as a medicinal plant had studied. Embelin has antibacterial and antiprotozoal properties and due to its cooling effects it is widely used in skin related ailments .it also improves the brain functioning and strengthens the nervous system. It is normalises the digestive activities .it helps in purifying the blood and also helpful in urine related problems. Benzoyl ester substituted derivatives of Embelin molecule also have the medicinal properties more or less then the parent Embelin molecule. In the present study we were prepared some Benzoyl ester substituted derivatives of Embelin. The synthesized compound were characterised and identified on the basis of elemental analysis, UV, FTIR, Mass and NMR spectroscopy and their antibacterial activities were studied against test organism viz B.subtilis gram positive,S.Aureus gram positive , C.Albicans Antifungal and E.Coli gram negative. The lipid lowering activities of prepared compounds were also studied in percentage [change in international unit (IU)] on pancreatic lipase inhibition.
Material and Methods
all chemicals used were of A.R Grade and purchased from S.D Fine and Lobachem chemicals (Mumbai) and were used further purification. The experimental part divided in to two parts
Isolation of Embelin
The parent molecule embelin (3-undecyl 2,5- dihydroxy ,1,4 –benzoquinone) was extracted and isolated from the fine powered berries of embelia ribes by the method explained by Gagan V.D & et al 8-9.the isolated Embelin further purified and used for the synthesis of Benzoyl ester substituted derivatives of Embelin.
Preparation of Benzoyl ester Substituted Derivatives of Embelin (EL-1 -EL-5)
Synthesis of 2,5-di-O-(4-tert.Dutylphenyl Carbonyl)-3-undecyl-1,4-Benzoquinone (EL-1)
in a three neck 100ml round bottom flask fitted with magnetic stirrer ,guard tube ,cooling bath was charged a mixture of 1.0g of embelin (0.003401 moles ,1 eq. ) and 1.61g of pyridine (0.02041 moles,6 eq.) in 30ml of Dichloro methane. The reaction mixture was stirred at room temperature for next 5 min. when clear solution of reaction mixture was obtained. The reaction mixture was kept in cooling bath (15-18 0C) and to that 2.7 g (2.7ml) of 4-tert.butyl benzoyl chloride (0.01306 moles,4.0 eq.) was added drop wise through dropping funnel over a period of 10 minutes. The reaction mixture was stirred at room temperature for next 24 hours. The reaction mixture was monitored by TLC for the completions of reaction.
Synthesis of 2,5-di-O-(4-Methyl Phenyl Carbonyl)-3-undecyl-1,4-Benzoquinone (EL-2)
in a three neck 100ml round bottom flask fitted with magnetic stirrer ,guard tube ,cooling bath was charged a mixture of 1.0g of embelin (0.003401 moles ,1 eq. ) and 1.61g of pyridine (0.02041 moles,6 eq.) in 30ml of Dichloro methane. The reaction mixture was stirred at room temperature for next 5 min. when clear solution of reaction mixture was obtained. The reaction mixture was kept in cooling bath (15-18 0C) and to that 2.1 g (1.75ml) of 4-methyl benzoyl chloride (0.01306 moles,4.0 eq.) was added drop wise through dropping funnel over a period of 10 minutes. The reaction mixture was stirred at room temperature for next 24 hours. The reaction mixture was monitored by TLC for the completions of reaction.
Synthesis of 2,5-di-O-(3-Bromo Phenyl Carbonyl)-3-undecyl-1,4-Benzoquinone (EL-3)
in a three neck 100ml round bottom flask fitted with magnetic stirrer ,guard tube ,cooling bath was charged a mixture of 1.0g of embelin (0.003401 moles ,1 eq. ) and 1.61g of pyridine (0.02041 moles,6 eq.) in 30ml of Dichloro methane. The reaction mixture was stirred at room temperature for next 5 min. when clear solution of reaction mixture was obtained. The reaction mixture was kept in cooling bath (15-18 0C) and to that 3.0 g (1.76ml) of 3-bromo benzoyl chloride (0.01306 moles,4.0 eq.) was added drop wise through dropping funnel over a period of 10 minutes. The reaction mixture was stirred at room temperature for next 24 hours. The reaction mixture was monitored by TLC for the completions of reaction.
Similarly the compound, 2,5-di-O-(3-chloro phenyl carbonyl)-3-undecyl-1,4-benzoquinone (EL-4) also prepared by the above method.
Synthesis of 2,5-di-O- Phenyl carbonyl-3-undecyl-1,4-benzoquinone (EL-5)
in a three neck 100ml round bottom flask fitted with magnetic stirrer ,guard tube ,cooling bath was charged a mixture of 1.0g of embelin (0.003401 moles ,1 eq. ) and 1.61g of pyridine (0.02041 moles,6 eq.) in 30ml of Dichloro methane. The reaction mixture was stirred at room temperature for next 5 min. when clear solution of reaction mixture was obtained. The reaction mixture was kept in cooling bath (15-18 0C) and to that 1.91 g (1.6ml) of benzoyl chloride (0.01306 moles,4.0 eq.) was added drop wise through dropping funnel over a period of 10 minutes. The reaction mixture was stirred at room temperature for next 24 hours. The reaction mixture was monitored by TLC for the completions of reaction.
Result and Discussion:
The 1H NMR spectra were recorded on Hitachi PerkinElmer spectrophotometer in CDCl3 Using TMS as internal standard. FTIR spectra (in 4000–450 cm–1range) of Embelin and its derivate were recorded in KBr pellets (2 mg / 200 mg KBr) using a FTIR PerkinElmer 1750 in department of chemistry, University of Mumbai, Mumbai. , the mass spectra were recorded on TOF-MS-ES[Micromass:Q-TOF micro(YA-105)] mass spectrophotometer.
1H NMR of compound ( EL-1) (300 MHz, CDCl3) δppm
1H NMR (300 MHz, CDCl3) δppm: 0.86 (t,3H,J=7.0 Hz),1.0-1.6 (m,36H),2.5(t,2H, J=7.5 Hz),6.75(S,1H),7.4-8.2 (m,8H)
In the 1H NMR spectra of compound (EL-1) a peak for side chain terminal methyl as triplet appears at δ 0.87 (J=7.5 Hz),the complex unsymmetrical multiplate in the region between δ 1.0-1.6 integrating for 36 protons were assign to two tertiary butyl and side chain methylene group (9 x –CH2) protons. The triplet at δ 2.5 (J=7.5 Hz) integrating for was assign to allylic methylene group. The singlet for aromatic ring proton appears at δ 6.75 and three sets of doublet resonating around δ 7.55, δ 7.55 and δ 8.09 corresponds to 2H, 2H and 4H.
FTIR Spectrum of compound( EL-1)
IR (KBr) cm-1:- 1745 (>C=O in ester), 2970 (CH), 1677(>C=O for α,β unsaturated carbonyl group), 1601-1595 ( Aromatic Stretching).
The disappearance of –OH group band at 3308 cm-1 as indicated in the parent embelin and appearance of ester carbonyl band at 1745 cm-1shows that reaction takes place at that site.
Mass spectrum of compound (EL-1): In the mass spectra of compound (EL-1) the molecular ion peak appears at m/z 615(M+) and the peak at m/z 600 was assign to characteristic aromatic side chain cleavage (M-CH3).
The UV, 1H NMR, FTIR and Mass spectra of synthesized compounds (figure-1 –figure-3) viz El, EL-2, EL-3, EL-4 and EL-5 were interpreted and tabulated in the Table-1 &Table-2.
Lipid lowering activity and Antibacterial activities:
The synthetic derivatives of Embelin were subjected to lipid lowering activity to get a more potent drug candidate in this domain. compounds (EL-1 –EL-5) 10-11 were dissolved in 1ml DMSO to obtain a 10mm stock solution and were vortexes to dissolution and stored at 4oC.the samples were prepared in 0.2M phosphate buffer,pH-8.0.this samples was used as test sample for all further assays.
Assays for lipase inhibitory activity:
Lipase assay
lipase assay was performed by method described by Winkler and Stukmann, 1979 with modification 12 assays was designed using a 96-well format. The substrate used in this assay was p-nitro phenol plamitate (Sigma cat.no. N-2752) 4.5 mg p-nitro phenol palmitate was dissolved in 200ml of DMF and volume made up to 10ml with 0.1M phosphate buffer at a conc. Of 5 mg/ml .the reaction mixture was incubated at 370C and optical density was measured at405 nm after incubation .enzyme activity was presented in the form of international unit (IU).
Lipase activity
One enzyme unit of lipase is defined as that quantity releasing 1nm of free phenol from substrate (p-nitro phenol plamitate) min-1/ml under the standard assay condition.
Lipase inhibition assay
The conc. Of synthetic analogues checked were 100μM and 200μM. The assay was similar to assay described above except 40μl of test sample was used instead of phosphate buffer in control. Optical density was measured at 0 hr and following incubation at 370C.
Enzyme inhibition
Enzyme inhibition was presented in the term of relative activity and % inhibition simply on the basis of change in international unit (IU).
IC50 calculation
IC50 of each compound was calculated manually from dose dependent graph (6.25 μM -200 μM) of analogue at the conc., where the % inhibition of lipase was measured as 50% in two near straight points .the value of IC50 was derived from linear regression. The value derived based on interpolated data. The IC50 values of synthesized compounds (EL- 1 to EL-5) shows better pancreatic lipase inhibition in comparison with starting material embelin (Table-3) and can act as a lead in developing new drugs in lipid altering domain.
Antibacterial spectrum of Embelin and its derivatives
Antibacterial activity of Embelin and its derivatives were tested against three bacterial strains viz Escherichia coli (gram-ve), staphylococcus aureus(gram+ve),bacillus subistilis(gram+ve) where antifungal activity was tested against the yeast candida albicans, stock solution of the compounds were prepared at 10 mg/ml concentration in DMF. Each compound was tested for its antibiotic activity at 250g concentration by agar diffusion method 13-15 against the microorganism mentioned above. The antibacterial and antifungal activity of the embelin derivatives were determined and compared with that of parent compound embelin(Table-3). While embelin showed antibacterial activity against B.Subtilis (gram + ve) and antifungal activity against C.albicans, the activity was completely knocked off in all compounds indicates that the role of the –OH group at these position in conferring the antibiotic activity.
Reaction Scheme
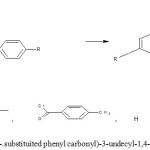 |
Schme 1: Synthesis of 2,5-di-O-(4-.substituited phenyl carbonyl)-3-undecyl-1,4-benzoquinone(EL-1,EL-2 & EL-5) Click here to View Schme |
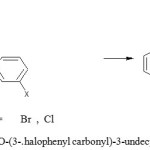 |
Schme 2: Synthesis of 2,5-di-O-(3-.halophenyl carbonyl)-3-undecyl-1,4-benzoquinone(EL-3 & EL-4)Click here to View Schme |
Table-1: 1HNMR (400 MHz, CDCl3) δppm and Mass spectra of Compounds
|
|
1HNMR peaks |
Mass spectrum Micromass: Q-TOF micro(YA-105) |
|||||||||||
|
Sr. No |
Compounds | Side chain Allylic -CH2 groupδppm | >C-H (1,4 benzoquinone moiety) proton singletδppm | Side chain -CH2 Protonunresolvedmultiplateδppm | Terminal -CH3Protontripletδppm | Aromatic ring 8H Protonδppm | Tert. Butyl groupProtons18H singlet δppm | Ar-CH3 Proton | -OH groupProtonBroadSinglet |
m/z value |
m/z value |
m/z value |
m/z value |
|
1 |
(EL) |
2.43 |
5.99 |
1.75-1.0 |
0.87 |
— |
—- |
—— |
7.66 |
294(M+) |
295(M++H) |
— |
— |
|
2 |
(EL-1) |
2.25 at J =7.0Hz |
6.75 |
1.6 |
0.86 at J =7.5Hz |
7.53-8.09 |
1.6-1.0 |
—– |
Absent
|
615(M+) |
600 (M-CH3) |
— |
—- |
|
3 |
(EL-2) |
2.5 at J =7.5Hz |
6.75 |
1.5-1.0 |
0.85 at J =7.5Hz |
7.3-8.10 |
—- |
2.3 |
Absent
|
601(M+) |
553 (100,M+ + Na) |
—- |
— |
|
4 |
(EL-3) |
2.51 at J =7.2Hz |
6.8 |
1.8-1.0 |
0.87 at J =7.2Hz |
7.45-8.3 |
— |
— |
Absent
|
731(M+) |
680(5,M+ + Na) | 682 (20,M++2 + Na) | 684(5,M++4 Na) |
|
5 |
(EL-4) |
2.5 at J =7.6Hz |
6.8 |
1.5-1.0 |
0.87 at J =6.9Hz |
7.4-8.2 |
— |
—- |
Absent
|
642(M+) |
593(9,M+ + Na) |
595 (3,M++2 + Na) |
—- |
|
6 |
(EL-5) |
2.5 at J =7.6Hz |
6.77 |
1.68-1.10 |
0.85 at J =7.5Hz |
7.42-8.22 |
— |
—- |
Absent |
573(M+) |
525(100,M+ + Na) |
105(for benzoyl ion) |
—- |
Table 2: UV λmax(methanol), FTIR (KBr) cm-1,Analytical and Physical Data of Compounds
|
UV Peaks in nm |
FTIR (KBr) peaks in cm-1 |
Elemental analysis Found (calculated) (%) |
|||||||||||
|
Sr. No |
Compounds | UV λmax(methanol)in nm | Tertiary-OH groupcm-1 | (>C=O for α,β unsaturated carbonyl group), cm-1 | (Aromatic –C-H Stretching) cm-1. | (>C=O in ester) cm-1 |
C |
H |
O |
X=Cl-,Br- | Molecular weight | MeltingPoint (0C) | Yield(%) |
|
1 |
(EL) |
284 |
3308 |
1613 |
3020 |
—- |
69.32 (69.36) | 8.84 (8.88) | 21.8 (21.76) |
—- |
294 |
142-143 |
— |
|
2 |
(EL-1) |
245(blue shift) |
Absent |
1677 |
3080 |
1745 |
76.14 (76.19) | 8.16 (8.20) | 15.66 (15.61) |
— |
615 |
Viscous mass |
69.2 |
|
3 |
(EL-2) |
233(blue shift) |
Absent |
1670 |
3080 |
1741 |
74.62 (74.69) | 7.16 (7.20) | 18.26 (18.09) |
— |
601 |
95-96 |
50.25 |
|
4 |
(EL-3) |
236(blue shift) |
Absent |
1673 |
3118 |
1742 |
56.32 (56.38) | 4.90 (4.88) | 14.60 (14.54) | 24.20 (24.32) |
731 |
98-99 |
60.10 |
|
5 |
(EL-4) |
236(blue shift) |
Absent |
1616 |
3080 |
1734 |
65.12 (65.15) | 5.59 (5.64) | 16.85 (16.8) | 12.41 (12.33) |
642 |
103-105 |
59.25 |
|
6 |
(EL-5) |
232(blue shift) |
Absent |
1674 |
1748 |
3078 |
74.02 (74.08) | 6.77 (6.82) | 19.16 (19.10) |
— |
573 |
98-100 |
50.88 |
NMR ,FTIR and Mass Spectrum of Compound (EL-1)
 |
Figure 1: NMR spectra of El-1 Click here to View Figure |
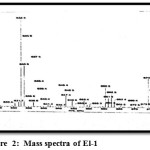 |
Figure 2: Mass spectra of El-1 Click here to View Figure |
 |
Figure 3: FTIR spectra of El-1 Click here to View Figure |
Table 3: Lipid lowering and antibacterial activities of compounds
|
Effect of compounds on pancreatic lipase inhibition |
Antibacterial spectrum of embelin and its derivatives |
||||||||||||
|
|
Compounds | % Inhibition(200μM) | % Inhibition(200μM) | IC50(μM) |
Quantity given (mg) |
Amount of DMF |
Conc.(mg/ml) | Amount used in wells(g) |
Zone of inhabitation (mm) |
||||
| B.Subtilis (gram + ve) | S.Aureus(gram + ve) | C.albicans(antifungal) | E.coli(gram – ve) | ||||||||||
|
|
Control (no inhibitor) | 0.00 | 0.00 | 0.00 |
— |
— |
— |
— | — | — | — | — | |
|
1 |
(EL-1) |
97.16 |
97.54 |
< 6.15 |
4.2 |
400 |
10 |
250 |
0 |
0 |
0 |
0 |
|
|
2 |
(EL-2) |
98.12 |
86.49 |
40.34 |
0.7 |
70 |
10 |
250 |
0 |
0 |
0 |
0 |
|
|
3 |
(EL-3) |
100.00 |
100.00 |
12.61 |
1.6 |
170 |
10 |
250 |
0 |
0 |
0 |
0 |
|
|
4 |
(EL-4) |
100.00 |
100.00 |
15.77 |
2.3 |
240 |
10 |
250 |
0 |
0 |
0 |
0 |
|
|
5 |
(EL-5) |
100.00 |
100.00 |
54.67 |
1.8 |
165 |
10 |
250 |
0 |
0 |
0 |
0 |
|
References
- Ajay,W.P.; Murcko,M.; J.med.Chem. 1998.,41, ,3314-3324.
CrossRef - Sadowski, J.; Kumbinyi, H. ; J.med.Chem. 1998.,41, ,3325-3329 .
CrossRef - Newmen,D.; Cragg,G.; Kingston,D.; The practice in medicinal chemistry, (Academic, London,) 2003, 91-109
CrossRef - Tyagi, R..D.; Tyagi, M..K.; Goyal, H..R.; Journal of research in Indian medicine 1987, 3, 130-132 .
- Rao, S.; Vilangin,v..;.Current Science, 1961 ,30,250-260.
- Gupta C.B.; Sanyal, S.N.; kanwar, U. ; as anticeptic effect of embelin ,a plant benzoquinone ,on male albino rats in vivo and vitro contraception,1986, 39, 307-320.
- Tripati, P.C.; Sengupta , J.; the role of embelia ribes in urinary tract infections international seminar on traditional medicine ,Calcutta , 1992,112-113 .
- Gagan, V.D.; Bamne, T.; Int.Res. J. Phrm. 2014,,2, 5.
- Mahesharan, S.; Sureshkumar, C.; Mohankumar, R.; International Journal of Pharma and BioScience, , 2013,4(3),116-123 .
- Yadav,R.P; Biotechnol. Appl.Biochem, 1998 ,28,,243-249 .
- Zimmermann, R.; Panzenbock, U.; Wintersperger, A.; lipoprotein lipase mediates the uptake of glycated LDL in fibroblasts ,endothiel cell,and microphges, diabetes 2001,50, 1643-1653.
- Antimicrobial – definitions from the Merrian –Webster online dictionary.www.merrian-webster.com/dictionary/Antimicrobial. Retrieved. 2009 .
- Theory of Agar diffusion methods bioassay R.K. finn Anal.Chem. , 1959, 31(6), 975-977.
CrossRef - Patange, A.N.; Yadav,U.M.; Desai, P.A.;International Letters of Chemistry, Physics and Astronomy ,2015, 52, 22-27.
- Patange, A.N.; Yadav, U.M,; Desai, P.A. ; Singare, P.U.; In world scientific News, 2015,4,, ,2392-2192.

This work is licensed under a Creative Commons Attribution 4.0 International License.









