Preparation of L-Arginine-Modified Silica-Coated Magnetite Nanoparticles for Au(III) Adsorption
Amaria1,2, Nuryono1 and Suyanta1
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, UniversitasGadjahMada, Sekip Utara, Yogyakarta 55281, Indonesia.
2Department of Chemistry, Faculty of Mathematics and Natural Sciences, UniversitasNegeri Surabaya, Jl. Ketintang, Surabaya 60231, Indonesia.
Corresponding Author E-mail: amaria@unesa.ac.id
DOI : http://dx.doi.org/10.13005/ojc/330146
L-arginine-modified silica-coated magnetite nanoparticles(Fe3O4/SiO2-GPTMS-Arg) have been synthesized by sol-gel process for adsorption of Au(III) ion in aqueous solution. Modification of L-arginine on silica coated magnetite through a coupling agent of 3-glycidoxypropyl-trimethoxysilane (GPTMS) was performed in avariousmole ratioof GPTMS:Arg 1:0; 1:1; 1:2 and 1:3.The products of Fe3O4/SiO2-GPTMS-Arg were characterized with XRD, FTIR, EDX, TGA, and Kjeldahl methods.The results showed that based on characterization data Fe3O4/SiO2-GPTMS-Arg has been successfully synthesized with the optimum mole ratio of 1:2. The optimum adsorption of Au(III) occurs at pH 3 and contact time of 60 min. The adsorption capacity followed Langmuir isotherm model was found0.638 mmol.g-1 for the Fe3O4/SiO2-GPTMS-Arg 1:2.Fe3O4/SiO2-GPTMS-Arg nanoparticles show a potential adsorbent for an effective Au(III) ion removal.
KEYWORDS:L-arginine; silica; magnetite; Au(III) ion adsorption
Download this article as:| Copy the following to cite this article: Amaria A, Nuryono N, Suyanta S. Preparation of L-Arginine-Modified Silica-Coated Magnetite Nanoparticles for Au(III) Adsorption. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Amaria A, Nuryono N, Suyanta S. Preparation of L-Arginine-Modified Silica-Coated Magnetite Nanoparticles for Au(III) Adsorption. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=28399 |
Introduction
In the era of modern technology today, gold is not only used for fixing, but also for any application such as household appliances, electronics, cosmetics, biosensors and for the treatment of cancer cells1,2. In industrial processes and manufacturing various electronic equipment,stages of coating, washing, chemical and mechanical polishingare necessary. At the stage of washing or rinsing waste water containing precious metal and hazardous ions is often generated3. Even thought the waste water produced only containing metal ions in low concentrations, those may be harmful to human health and other living creatures. Therefore those are necessary to be managed and removed from the waste water.
A variety of techniquesfor wastewater treatment and separation of precious metal ions have been reported, such as chemical precipitation4, ion exchange5, adsorption6,7, membrane filtration8, and electrochemical7,9. Among of the above techniques, adsorption has been widely used since it is proven to be more effective and economical10 because the design and operation are flexible.The adsorption is a reversibleprocess so thatthe adsorbent can be regenerated through desorption process forreuse11.Efforts to develop the adsorbent containingselective functional groups have been conducted12,13, but to recoverprecious metal ions and removal of heavy metal ion are still challenge. This is due to the capacity and selectivity towards targetedmetal ions low and high cost 14. Therefore, efforts to probe and develop a new adsorbent or method still continue.
The use of organic materials, such as organic polymers, cellulose, algae, and yeast biomass as adsorbent has aweakness, such as swelling, very sensitive to chemicals and lower its mechanical stability. In other hands, support inorganic solids, such as clay, zeolite, metal oxides and silica are preferable due to relatively resistant in term of physicochemistryand good selectivity. Silica gel has attracted the attention of researchers, because ofhigh chemical andmechanical stabilitiesand low swelling15. In addition, the silica containing a lot of silanolgroups on the surface makes easy to be modified with theorganic functional group.
Modification of silica gel with amine groups has been reported to be highly efficient in removing of Cu(II), Ni(II), Pb(II), Cd(II), Zn(II) and Hg(II) ions in aqueous medium16,17. Aminoguanidinegroups have been used asa modifier of adsorbentfrom the tannin extract of persimmon for recovery ofAu(III) ion in acidic media18. Therefore, ligands with amine functional groups are very effective to adsorb precious metal ions19.Modification of the silica gel with the active site of mercapto (-SH) and an amine (NH2) hasbeen reported by Nuryono et al.20for Au(III) ion adsorption. The hybrid of amino-silica adsorbedtheAu(III) ion in similar amount (98.77%) tothatof mercapto-silica (96.67%) at the pH 4.In thatcondition, the amine groups of the amino-silica hybridsurface are protonated to form cationic groups-NH3+ and interact with anionic species of Au(III) ion, AuCl4–. The interaction between Au(III) ion with amino-silica hybrid might happenthroughionic interactions.While the mercapto-silica hybrid, the S atoms donate the electron pair to Au(III) ions to form complexes using covalent interactions.
Nowadays, magnetic separation is considered as an effective method for the recoverypreciousmetalsand removal of heavy metal ions. Magnetite nanoparticles (Fe3O4) modified with functional groups such as dendrimer21, dimethylamine22, dimercaptosuccinicacid23, and 3-aminopropyltrietoksisilan24has been tested to be capable of removingheavy metal ions due to the high adsorption ability, time is short and separation of the adsorbent from the solution is easy because it uses an external magnetic field. The active site which contains a nitrogen or a sulfur atom is selectively high for the precious metal ion according to thehard soft acid base (HSAB) theory25. The magnetite nanoparticles which modified by thiourea (SC(NH2)2)26have been reported to be capable of separating and recovering precious metal ions from acid solution.
L-arginine molecules have aflexible chain of guanidine groups with pKa of 12.5.27 Guanidine (-NHCNHNH2) groups with apositivechargein acidic or neutral conditions leads to adsorb AuCl4– anion from species of Au(III) ion18.In this research, we have prepared L-arginine modified silica coated on magnetite nanoparticles surface using a linker of 3-glycidoxypropyltrimethoxysilane (GPTMS) in thesol-gel system. The effectof amole ratio of L-arginine to GPTMS on the chemical stability, the content of nitrogen and the adsorption propertiesfor adsorption of Au(III) ions in aqueous solution has been evaluated.
Materials and Methods
Material
In this study,we used chemicals such asiron (II)/(III) oxide, 3-glycidoxypropyl-trimethoxysilane (GPTMS) (≥98%) from Sigma-Aldrich. Sodium silicate (25-27%), L-arginine powder, ethanol absolute (99.5%), HCl 37%, NaOH, HNO3 65%, thiourea, Au standard solutionwere purchased from Merck used without previous treatment. Precious metal of gold was purchased from PT. Aneka Tambang.
Preparation of L-Arginine-Modified Silica-Coated Magnetite Nanoparticles(Fe3O4/SiO2-GPTMS-Arg)
In a beaker polyethylene, magnetite0.4 gwas added with 10 mLof distilled waterandsonicatedfor 5minutes. After that,the suspensionwas added12 mLof 2.8MNa2SiO3 solution andsonicated. After the suspensionstirred mechanically for 30minutes, it was added1.13mL ofGPTMSand stirred it about 30 minutes. Furthermore, the suspension was added with5mmol L-argininein order the mole ratio of GPTMS to L-arginine 1:1, stirredfor 60 minutesand 2MHCladdeddropwiseuntilpH7 and formed a gel. After the gelleft in one night, gelwashedwith aquamineraland flushed with ethanol three times, and dried at 65°C in the oven until its weight of constant.Analogue work was conductedvarying the mole ratioof GPTMS to L-arginine according to Table 1.
Characterization
The functional groupsinsamples wereidentifiedwith FTIR spectrophotometer (Shimadzu FTIR 8400S) with KBrpellettechnique in wave number400-4000 cm-1. Powder XRD pattern of samples was compiled on Bruker D8 Advance 206276 (Sourceusedradiation ofCu-Kα (λ 0.154 nm) on40kVvoltage and30mAcurrent. X-ray diffraction patterns were scanned at a2θrange of3-80°inscanspeed5°/min.The nitrogen content ofsamples wascounted with energy dispersive X-ray (EDX Carl Zeiss 9 EVO MA 10 series 1454)and Kjeldahl method. TGA was carried out on Mettler Toledo witharate of heating20°C per minute,at temperature 40-800°C. Absorption atomic spectrophotometer Analyst 100 Perkin Elmer apparatus was used to define thecontent of metal ion in solution. Niobium magnet was used for the magnetic separation. The measurement of thepH solution was conducted with a pH meter (pH/ion 510 EutechOakton).
The chemical stability wastestedby mixing25 mg of samplewith 25 mLHClsolution 1 M and shaking for3hours. After that, it was left overnightat a room temperature. The filtrate was dividedfrom the mixtureby an external magnetic force anddissolvedironwas determined by atomic absorption spectroscopy method28,29.
Table 1: Mole ratio of GPTMS to L-arginine on preparation of the L-arginine modified silica-coated magnetite nanoparticles
|
mmol GPTMS |
mmol L-arginine |
Mole ratio GPTMS/L-arginine |
Sample Label |
|
5 |
0 |
1:0 |
Fe3O4/SiO2-GPTMS-Arg 1:0 |
|
5 |
5 |
1:1 |
Fe3O4/SiO2-GPTMS-Arg 1:1 |
|
5 |
10 |
1:2 |
Fe3O4/SiO2-GPTMS-Arg 1:2 |
|
5 |
15 |
1:3 |
Fe3O4/SiO2-GPTMS-Arg 1:3 |
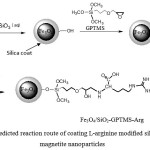 |
Figure 1: Predicted reaction route of coating L-arginine modified silica on magnetite nanoparticles |
Adsorption of Au(III)
Adsorption of Au(III) on Fe3O4/SiO2-GPTMS-Arg was conducted by theeffect of pH, adsorption time and concentration of Au(III) ions. Adsorbent 10 mg was addedto10 mL of Au(III) solution in a batch system ofa polyethylene bottle at a room temperature (302K). Themixturewas shaken at 350 rpm for certain time and the adsorbent was separated with an external magnet. The concentration of Au(III) ionnot adsorbed in thesupernatantwas measured by Atomic Absorption Spectrophotometer (AAS). A number of Au(III) ions adsorbed was calculated by the difference between the initial and final concentrationof Au(III) in the supernatant usingEq. (1)

q is Au(III) ions adsorbed (mmol.g-1); C0and Ce are initial and final concentrations of Au(III) (mmol.L-1), respectively. V is volume (L) of the Au(III) solution and Wisthe adsorbent mass (g).
The pH of medium was controlledby0.1 M HCl and/or 0.1 M NaOH solution to reach 1-5, the effect of concentration of Au(III) ion was carried on 25-250 mg L-1 and the variationof contact time was observed at the range of 5-180 min. The models of Langmuir and Freundlich isotherm were applied to evaluate data of the effects ofinitial concentrationand the models of somekinetics reaction are used to verify the appropriate adsorption rate constant.
The experiment of desorption was conducted by using thiourea solution in 1 M HCl- HNO3. The firstly, 200 mg of adsorbent was added with 50 mL100 mg.L-1Au(III) at optimum pH for 60 minutes. After the mixture was shakenand separated with a magnet, thesupernatant was analyzed by AAS to decide the content of Au(III) not absorbed. The adsorbentloadedwith Au(III)was washed byaquamineraland dried at 65°C to get theweight of constant and stored in a desiccator overnight before desorption process. The adsorbentloadedwith Au(III) was added with 10 mL of H2O, and then was shaken for 10 min and supernatantwas separated with anexternal magnet. The supernatant was analyzed by AAS to decide thecontent of Au(III) that leached with H2O. The adsorbent-loaded was added with 10 mL of 0.5 M thiourea solution in 1M HCl. Then the mixture was shakenfor 8 h andseparated with a magnet, andthe Au(III) ion releasedwas measured its concentration. The desorptionwas repeated three times using the same eluent. Desorption data of Au(III) was calculated with Eq 230:
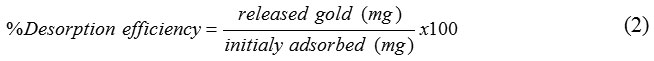
Results and Discussion
Characteristic of Fe3O4/SiO2-GPTMS-Arg
Functional groups
The FT-IR spectra of silica-coated magnetite nanoparticles with and without L-arginine modification are showed in Fig 2. All spectra have a peak at 568.96 cm-1. The peak is ascribed to Fe-O mode of magnetite31. Peaks3446.56 cm−1 and 1645.17 cm−1 are associated the O-H stretching and bending vibration, respectively (Fig 2a).The characteristic peak of silica is observed ataround of 1100 cm−1related to the Si-O(Si-O-Si)asymmetricstretching vibration32. In all spectra, the peak at 796.55 cm-1 is observed, assigned to symmetric stretching of siloxane Si-O(Si-O-Si), while the band around 460.96cm−1 corresponds to the Si-O-Si or O-Si-O bending modes33. This suggests that silica has been coated on the magnetite surface34. FromFig 2(b-d) can be seen peaks at 3415.70, 3443.06 and 3411.84 cm-1 ascribing the presence of amino groups. Those peaks are apparentbroader than that of Fig 2a due to overlappingwith O-H stretching vibration.
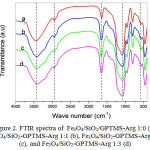 |
Figure 2: FTIR spectra of Fe3O4/SiO2/GPTMS-Arg 1:0 (a), Fe3O4/SiO2-GPTMS-Arg 1:1 (b), Fe3O4/SiO2-GPTMS-Arg1:2 (c), and Fe3O4/SiO2-GPTMS-Arg 1:3 (d) |
Peak around 2939.31 cm-1is appointedto thestretching vibration of C-H asymmetric32, fromGPTMS and L-arginine. The peak at 1645.17 cm−1 (Fig 2a) is related the O-H (Si-OH) bending vibration (due to condensation of GPTMS), while the peak at 1654.81, 1662.52, and 1656.74 cm−1 (Fig 2b-d)are related to the N-H bending vibration18,35. It indicates that the coupling agent of GPTMS and modifier of L-arginine have been modified on silica-coatedmagnetite nanoparticles surface.
Crystalline Structure
The X-ray diffraction patternof the L-arginine modified silica coating on magnetite nanoparticles surface synthesized with various mole ratios of GPTMS to L-arginineare presented in Fig. 3. The observationof X-ray diffraction pattern for four samples seemscharacteristic peaks of magnetite confirmed byJCPDS 19-0629with the index field (220), (311), (400), (511) and (440)36, and hence it denote that the cubic crystal phase of magnetite is stand after coating with L-arginine-modified silica37. The presence of SiO2amorphous phase is showed by specific 2θ at 22 degrees38(Fig.3b, c, d, and e) according to JCPDS No. 46-1045.
 |
Figure 3: XRD patterns ofFe3O4/SiO2-GPTMS-Arg 1:0 (a) Fe3O4/SiO2-GPTMS-Arg1:1(b),Fe3O4/SiO2-GPTMS-Arg1:2(c), and Fe3O4/SiO2-GPTMS-Arg 1:3 (d) |
Chemical stability
Magnetite powder is readilydissolvedin acidic solution39. Silica coating may stabilize magnetite from an acidic medium34,39,40. In this work, the magnetite nanoparticles werecoated with silica and added amodifier of L-arginine. The samples of Fe3O4/SiO2-GPTMS-Arg obtained were mixed with hydrochloric acid 1 M for 1day. Iron leachedfrom the sample was determined with AAS. The result showed that the acid solution leached iron from the coated magnetite samples in a range of 0.235-0.344 mmol g-1. It is much lower than the amount of iron leached from uncoated magnetite (1.09 mmol g-1).
The content of nitrogen element
L-arginine as a modifier to thesilica coated magnetite contains amine groups which may react wellwith Au(III) in solution.Based on the FTIR spectra in Fig. 2 seem that coated magnetite contains amine group -NH from L-arginine.In addition, we also confirmed the presence of nitrogen element from L-arginine usingthe EDX and Kjeldahl methods41 and the result is expressed in Table 4. The content of nitrogen in sample synthesized with amole ratioof GPTMS to Arginine 1:2 and 1: 3 analyzed with EDX and Kjeldahl methodsare relativelysimilar and higher than in sample synthesized with the ratio 1:1. However, the content of nitrogen based on the theoretical calculation is much greater than result from theexperiment. This difference occurs probablysince not all arginines are bonded on the surface. The presence of nitrogen in all samples supports the success of modification of arginine on silica coated magnetite.
Table 4: The content ofnitrogen inL-arginine modified silica-coated magnetite nanoparticles based on measurements of EDX, Kjeldahl method, and the theoretical calculation
|
Method |
wt% N |
||
|
Fe3O4/SiO2-GPTMS-Arg 1:1 |
Fe3O4/SiO2-GPTMS-Arg 1:2 |
Fe3O4/SiO2-GPTMS-Arg 1:3 |
|
|
EDX |
3.28 |
4.86 |
4.07 |
|
Kjeldahl |
2.478 |
3.610 |
3.958 |
|
Theoretical calculation |
6.189 |
10.386 |
13.421 |
Thermogravimetric Analysis
Thermogravimetric analysis (TGA) was conducted on four samples of magnetite coated with silica-arginineat temperaturesfrom 40°C- 800°C, with the heating rate of 20°C per minute. Fig.4 shows that there are four stagesof weight lossin the TGA curve. The first stage, degradation occurs at a temperature of 40-100°C, the second stage of 100-250°C, the third stage of 250-500°C, and the fourth stage of 500-800°C. In Fig. 4 is observed that all four samples of coated magnetite lostthe weight at atemperature of 40-100 °C (<100 °C).The weight loss is related to the removal of water molecules adsorbed physically, which removal of water molecules is continued at the temperature of 100-220°C 42,43. Fig.4 shows that the weight lossin the second stage at that temperature range (100-250°C), for Fe3O4/SiO2-GPTMS-Arg 1:0, 1:1, 1:2, 1:3is 3.279; 3.384; 4.306 and 4.707%, respectively.
At the temperature range of 250-500°C, TGA curves of Fe3O4/SiO2-GPTMS-Arg 1:0 the weight loss is 9.208%. The weight loss is linked to the decay of the organic part of a silica network, as reported Shajesh et al.43. Another reason of the weight loss is a dehydration and dehydroxylation reaction of silanol (vicinal, geminal, and combinations) in the silica42,44. Meanwhile, Fe3O4/SiO2-GPTMS-Arg 1:1, 1:2, and 1:3 at the temperature range of 250-500°C loosedthe weightof7.570, 18.549, and 17.577%, respectively. In that temperature, the decomposition of organic parts43,45, including L-arginine bound to the silica networkproduces CO and CH4. At higher temperature than 500°C NH3 is released. At atemperature rangeof 250-500 °Cshowthat the percentage of the weight loss is higher for L-arginine-modified silica-coated magnetite sample preparedwith greatermole ratio. This phenomenon can be explained that smaller size nanoparticleshave thelarger surface areaso that it can bind arginine on the surface in larger number, and the percentage of weight loss is greater46,47.
At atemperature rangeof 500-800 °C (inthe fourth stage),the weight loss is related to the silanol dehydroxylation, especially silanol ingeminal position42. Thermal decomposition of all sample at this temperature are decreasing, this shows that all the decomposition stopped. In addition, at temperature 500°C, the magnetiteis changed into hematite48.
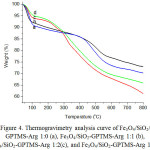 |
Figure 4: Thermogravimetry analysis curve of Fe3O4/SiO2/GPTMS-Arg 1:0 (a), Fe3O4/SiO2-GPTMS-Arg 1:1 (b), Fe3O4/SiO2-GPTMS-Arg 1:2(c), and Fe3O4/SiO2-GPTMS-Arg 1:3(d). |
Adsorption properties
Effect of medium pH on the Au(III) ion adsorption
The experimental resultto study the effect of pHon is presented in Fig.5 showing that for all investigated adsorbents adsorption of Au(III) reaches optimum at pH 3. In the highly acidic condition (at pH 1-2), the adsorption is very lowand at higher pH (above 3), the adsorptiondecreases. AdsorbentsFe3O4/SiO2-GPTMS-Arg 1:1, 1:2, and 1:3 show relatively similar adsorption capability (61.95, 67.45, and 66.88 mg g-1,respectively). Fe3O4/SiO2-GPTMS-Arg 1:0 gives very low adsorption capability. It is associated with theactive site in the magnetic adsorbent surface, without L-arginine,the probable active site playing a role in adsorption is only -OH of GPTMS. Au(III) in solution at pH 2-3 is dominant as a stable complex ion [AuCl4]–and at increased pH, chloride ion in the complex [AuCl4]– is replaced by OH– ions. At a pH of 4-9 in [AuCl3OH]–[AuCl2OH2]–[AuClOH3]–are formed and at pH higher than 9 the [AuOH4]–form dominates49.In the acidic condition amino groups (NH2) of arginine on silica-coated magnetite nanoparticlessurface undergo protonation to generate positively charged groups(-NH3+).Those groups mayinteracts electrostatically with AuCl4–, a species of Au(III) in acidity. As seen in Fig. 5 the adsorption of Au(III) at pH 3 is observedmaximum.The interactionbetween thesurface of Fe3O4/SiO2-GPTMS-ArgandAu(III) metal ionsin acidic conditions is illustrated hypothetically in Fig.6.
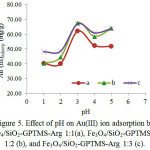 |
Figure 5: Effect of pH on Au(III) ion adsorption by Fe3O4/SiO2-GPTMS-Arg 1:1(a), Fe3O4/SiO2-GPTMS-Arg 1:2 (b), and Fe3O4/SiO2-GPTMS-Arg 1:3 (c). |
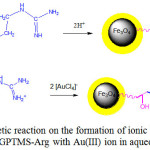 |
Figure 6: Hypothetic reaction on the formation of ionic bonds between Fe3O4/SiO2-GPTMS-Argwith Au(III)ion in aqueous solution. Click here to View figure |
Adsorption isotherm of Au(III)
The adsorption isotherms of Au(III) on four magnetic adsorbentswere tested withthe effect ofinitial concentrations of Au(III) (25-250 mg/L) at pH 3. The adsorption experimental data were evaluated by Langmuir and Freundlich isotherm models34,50 expressed in mathematical equation (Eq.3and 4).

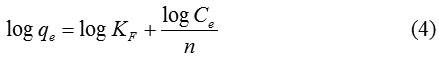
where qe and Ce are the amount Au(III) adsorbed and concentration of Au(III) at equilibrium, qm is adsorption capacity and KL is the constant of Langmuir relating tothe binding sitesaffinity. The value of qm obtained from slope and KL found from the intercept of a plotof Ce/qevsCe.KF.The constantof Freundlich is related tothe adsorptioncapacity and n is Freundlich exponent linked to the intensity of adsorption. The values of KF and nare obtained from slope and intercept of plot log qe vs log Ce. The adsorption process according to Langmuir isotherm isassumed as monolayer and occurs on the sites of the homogenous surface. While the Freundlich isotherm assumed that the adsorptionis heldon the sites of the heterogeneous surface. The adsorption data of Au(III) on Fe3O4/SiO2-GPTMS-Arg 1:0-1:3 are shown in Fig 7 and Table 5 presents the Langmuir and Freundlich isotherm constants for adsorption of Au(III) on Fe3O4/SiO2-GPTMS-Arg 1:0-1:3. The linear coefficient (R2) values for Langmuir isotherm models, in general, are higher than 0.99. This indicates that the Langmuir isotherm fits for the experimental data of Au(III) adsorption.
From Table 5 can be seen that the value of the adsorption capacity of Fe3O4/SiO2-GPTMS-Argincreases with the molenumber of L-arginine added, namely 0.209; 0.544; 0.638; 0.654 mmol/g, which correspondto the nitrogen content.Thisindicates thatfunctional group participates in the adsorptionis–NH3+.The adsorbent without L-arginine (Fe3O4/SiO2-GPTMS-Arg 1:0) giveslowest adsorption capacityand the adsorbentscontaining L-arginine showcapacity 1.87-3.13 times higher. The value of DG° (Table 5) are higher than 21 kJ/mol51indicating the involvement of chemical interaction, asillustrated in hypothetic reaction(Fig 6).
Adsorption kinetics
Study on the kinetics of Au(III) adsorption was conducted by interacting with 50 mg L-1 Au(III) solution at various contact times (5-180 min), pH 3 and room temperature (302 K). The adsorption data is presented in Fig. 8. It is observed that in the first five minutes adsorption occurs rapidly, followed by slow inclining and lastly reached equilibrium after 60 minutes. It was associated with a lot number of sites on the coated magnetite adsorbent surface available for Au(III)in the earlyreactionand finalized with equilibrium after saturation.
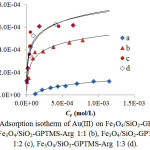 |
Figure 7: Adsorption isotherm of Au(III) on Fe3O4/SiO2-GPTMS-Arg 1:0 (a), Fe3O4/SiO2-GPTMS-Arg 1:1 (b), Fe3O4/SiO2-GPTMS-Arg 1:2 (c), Fe3O4/SiO2-GPTMS-Arg 1:3 (d). |
Table 5: Adsorption isotherm parameters of Au(III) on Fe3O4/SiO2-GPTMS-Arg
|
|
Langmuir |
Freundlich |
|||||
|
Adsorbent |
qmax (mmol/g)
|
K (10-3) (L.mol-1)
|
DG° (KJ. mol-1) |
R2 |
KF(10-2) (mol/ g-1) |
n |
R2 |
| Fe3O4/SiO2/ GPTMS-Arg 1:0 |
0.209 |
1.264 |
17.933 |
0.968 |
22.0 |
0.94 |
0.831 |
| Fe3O4/SiO2/ GPTMS-Arg 1:1 |
0.544 |
17.467 |
24.525 |
0.995 |
0.61 |
2.98 |
0.907 |
| Fe3O4/SiO2/ GPTMS-Arg 1:2 |
0.638 |
48.042 |
27.066 |
0.999 |
0.78 |
3.21 |
0.827 |
| Fe3O4/SiO2/ GPTMS-Arg 1:3 |
0.654 |
33.671 |
26.174 |
0.997 |
1.00 |
2.87 |
0.890 |
The reaction kinetics models of pseudo-first-order and pseudo-second-order were applied to evaluate the adsorption of Au (III) on the adsorbents, two common models for adsorption of metal ions on asolid adsorbent. The reaction kinetics models of pseudo-first-order and pseudo-second-order are stated inEq (5) and (6), respectively52,53.
![]()

qeand qtare the amounts of adsorbed metal ion at equilibrium and anytime, respectively (mmol. g-1), and k1(min-1) and k2 (g.mmol-1.min-1) are the rate constants of pseudo-first-order and pseudo-second-order. The rate constants can be determined from the linear curve plot of (ln qe-qt)against t andt/qt against t to pseudo-first order and pseudo-second order reactions, respectively. The result of calculation is presented in Table 6, showing that the adsorption kinetics model of pseudo-secondorderis more suitablewith the higher linear coefficient(R2) value (0.999) than pseudo-first-order one. This shows that the adsorption system involves the exchange of electrons between the magnetic adsorbent and Au(III) ion in aqueous solution37,53.
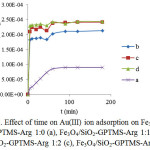 |
Figure 8: Effect of time on Au(III) ion adsorption on Fe3O4/SiO2-GPTMS-Arg 1:0 (a), Fe3O4/SiO2-GPTMS-Arg 1:1(b), Fe3O4/SiO2-GPTMS-Arg 1:2 (c), Fe3O4/SiO2-GPTMS-Arg 1:3 (d). Click here to View figure |
Table 6: Adsorption rate constant of Au(III) on adsorbents based on the kinetics modelsof pseudo-first and pseudo-secondorder
|
Sample |
Kinetics model |
|||
|
Pseudo-first order |
Pseudo-second order |
|||
|
k (min-1) |
R2 |
k (g.mmol-1.min-1) |
R2 |
|
|
Fe3O4/SiO2-GPTMS-Arg 1:0 |
0.0708 |
0.9665 |
0.3701 |
0.9830 |
|
Fe3O4/SiO2-GPTMS-Arg 1:1 |
0.0255 |
0.8811 |
1.3969 |
0.9988 |
|
Fe3O4/SiO2-GPTMS-Arg 1:2 |
0.0380 |
0.9792 |
1.7721 |
0.9991 |
|
Fe3O4/SiO2-GPTMS-Arg 1:3 |
0.0352 |
0.8711 |
3.5172 |
0.9997 |
Desorption
The Au(III) ion adsorbed on Fe3O4/SiO2-GPTMS-Arg was recovered by sequence desorption experiments. Desorption of precious metalsby using a thiourea solution, HCl, thiourea-HCl, HNO3,thiourea-HNO3has been reported by previous researchers22,26,30,54,55. In this work, desorption of Au(III) adsorbed onFe3O4/SiO2-GPTMS-Arg 1:2(25.068 mg/g) was carried outwithin the different composition of eluent at a room temperature as presented in Table 7.Each fraction (10 mL) of desorption, the concentration of Au(III) desorbed was determined with AAS. As shown in Table 7, desorption using1 M thiourea-0.5 M HClgives the highest desorption percentage (75.2997%). However, this result has not satisfied yetand theinvestigation is still going on to reach 100 % of recovery.
Table 7: The desorption data ofAu(III) loaded onFe3O4/SiO2-GPTMS-Arg 1:2with different eluents.
|
Desorber eluent |
Fraction/Eluent |
Au(III) desorbed (%) |
Desorption Total (%) |
|
0.5 M Thiourea-1 M HCl |
10 mL Aquademineral |
0.5231 |
60.8431 |
|
10 mLThiourea -HCl |
57.8411 |
||
|
10 mL Thiourea -HCl |
2.4789 |
||
|
10 mL Thiourea -HCl |
0 |
||
|
1 M Thiourea-0.5 M HCl |
10 mL Aquademineral |
0 |
75.2997 |
|
10 mLThiourea -HCl |
54.7049 |
||
|
10 mLThiourea -HCl |
20.4661 |
||
|
10 mLThiourea -HCl |
0.1287 |
||
|
0.5 M Thiourea-1M HCl-1M HNO3 (3:1) |
10 ml Aquademineral |
0 |
64.3061 |
|
10 mLThiourea – HCl-HNO3 |
58.4736 |
||
|
10 mLThiourea – HCl-HNO3 |
5.6993 |
||
|
10 mLThiourea – HCl-HNO3 |
0.1332 |
Conclusion
In conclusion, L-arginine-modified silica-coated magnetite nanoparticles (Fe3O4/SiO2-GPTMS-Arg) have been successfully synthesized using 3-glycidoxypropyltrimethoxysilane as the coupling agent via a sol-gel process with the optimum mole ratioof GPTMS to arginine of 1:2.Adsorption of Au(III) on Fe3O4/SiO2-GPTMS-Arg 1:2 occurredoptimally at pH 3, followed themodelof pseudo-second-orderwith arate constant of1.77 g. mmol-1.min-1 and fitted to themodel of Langmuir isotherm with the capacity of 0.64mmol.g-1.The further investigation to findthe best desorption technique in which Au(III) may be leached from adsorbent 100 % is still going on, and it is expected that magnetic material produced may be useful for recovery of precious metals from both industrial waste water and precious metal mining samples.
Acknowledgement
We would like to give the highest gratitude to the Ministry of Research, Technology and Higher Education of the Republic of Indonesia through the Research Grant Disertasi Doktor2016 and Postgraduate Scholarship for the financial support.
Reference
- Widmer, R.; Oswald-Krapf, H.; Sinha-Khetriwal, D.; Schnellmann, M.; Böni, H. Environ. Impact Assess. Rev.2005, 25 (5 SPEC. ISS.), 436–458.
- Guix, M.; Carbonell, C.; Comenge, J.; García–Fernández, L.; Alarcón, A.; Casals, E. Contrib. to Sci.2008, 4 (2), 213–217.
- Cui, J.; Zhang, L. J. Hazard. Mater.2008, 158 (2), 228–256.
CrossRef - Fu, F.; Wang, Q. J. Environ. Manage.2011, 92 (3), 407–418.
CrossRef - Dabrowski, A.; Hubicki, Z.; Podkoscielny, A.; Robens, E. Chemosphere2004, 56, 91–106.
CrossRef - Lopes, C. B., Lito, P. F., Cardoso, S. P., Pereira, E., Duarte, A. C., and S. C. M. Ion Exchange Technology II; Inamuddin and M. Luqman, Ed.; Springer Science+Business Media B.V, 2012; Vol. 10.
- Cornell, R. M.; Schwertmann, U. Introduction to the Iron Oxides, 2nd Editio.; WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim All: Darmstadt, 2004.
- Camarillo, R.; Llanos, J. Sep. Purif. Technol.2010, 70, 320–328.
CrossRef - Kurniawan, T. A.; Chan, G. Y.; Lo, W.-H.; Babel, S. Chem. Eng. J.2006, 118 (1), 83–98.
CrossRef - Ekmekyapar, F.; Aslan, A.; Bayhan, Y. K.; Cakici, A. J. Hazard. Mater.2006, 137 (1), 293–298.
CrossRef - Pan, B.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q.; Zheng, S. Chem. Eng. J.2009, 151 (1–3), 19–29.
CrossRef - Ngah, W. W.; Fatinathan, S. J. Environ. Manage.2010, 91 (4), 958–969.
CrossRef - Liu, C.-C.; Wang, M.-K.; Chiou, C.-S.; Li, Y.-S.; Lin, Y.-A.; Huang, S.-S. Ind. Eng. Chem. Res.2006, 45 (26), 8891–8899.
CrossRef - Hubicki, Z.; Wo\lowicz, A. Hydrometallurgy2009, 96 (1), 159–165.
CrossRef - Jal, P. K., Patel, S., Mishra, B. K. Talanta2004, 62 (5), 1005–1028.
CrossRef - Aguado, J.; Arsuaga, J. M.; Arencibia, A.; Lindo, M.; Gascón, V. J. Hazard. Mater.2009, 163 (1), 213–221.
CrossRef - Radi, S.; Basbas, N.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Cazier, F. Prog. Nanotechnol. Nanomater.2013, 2, 108–116.
CrossRef - Gurung, M.; Adhikari, B. B.; Morisada, S.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. Bioresour. Technol.2013, 129, 108–117.
CrossRef - Adhikari, C. R.; Parajuli, D.; Inoue, K.; Ohto, K.; Kawakita, H.; Harada, H. New J. Chem.2008, 32 (9), 1634–1641.
CrossRef - Nuryono, N.; Indriyanti, N. .; Manuhuntu, J. .; Narsito; Tanaka.S; Rosiati, N.; Rusdiarso, B.; Sakti, S. C.; Tanaka, S. Malaysian J. Anal. Sci.2013, 3 (1), 244–254.
- Chou, C.-M.; Lien, H.-L. J. Nanoparticle Res.2011, 13 (5), 2099–2107.
CrossRef - Zhou, L.; Xu, J.; Liang, X.; Liu, Z. J. Hazard. Mater.2010, 182 (1–3), 518–524.
CrossRef - Yantasee, W.; Warner, C. L.; Sangvanich, T.; Addleman, R. S.; Carter, T. G.; Wiacek, R. J.; Fryxell, G. E.; Timchalk, C.; Warner, M. G. Environ. Sci. Technol.2007, 41 (14), 5114–5119.
CrossRef - Lin, Y.; Chen, H.; Lin, K.; Chen, B.; Chiou, C. J. Environ. Sci.2011, 23 (1), 44–50.
CrossRef - Pearson, R. G. J. Chem. Educ.1968, 45 (9), 581.
CrossRef - Lin, T. L.; Lien, H. L. Int. J. Mol. Sci.2013, 14 (5), 9834–9847.
CrossRef - Nelson, D. L., Cox, M. M. Lehninger Principles of Biochemistry, Fifth Edit.; Katherine Ahr, Ed.; W. H. Freeman and Company: New York, 2008.
- Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. J. Colloid Interface Sci.2010, 349 (1), 293–299.
CrossRef - Nuryono, N.; Rosiati, N.; Rusdiarso, B.; Sakti, S. C.; Tanaka, S. Springerplus2014, 3 (1), 515.
CrossRef - Amaria, A.; Suyono, S.; Sugiharto, E.; Rohmah, A. N. Indones. J. Chem.2010, 10 (2), 177–183.
- Casillas, P. E. G.; Gonzalez, C. A. R.; Pérez, C. A. M. Infrared Spectroscopy of Functionalized Magnetic Nanoparticles; Theophile, T., Ed.; Intech: Rijeka, Croatia, 2012.
CrossRef - Silverstein, M. R.; Webster, F. X.; Kiemle, J. D. Spectrometric Identification of Organic Compounds, Seventh Ed.; Brennan, D., Ed.; John Wiley & Sons, Inc.: Hoboken United States of America, 2005.
- Ahangaran, F.; Hassanzadeh, A.; Nouri, S. Int. Nano Lett.2013, 3, 3–7.
CrossRef - Nuryono, N.; Muliaty, E.; Rusdiarso, B.; Candra, S.; Sakti, W.; Tanaka, S. J. Ion Exch.2014, 25 (4), 114–121.
CrossRef - Kumar, S.; Rai, S. B. J. Pure Appl. Phys.2010, 48 (April), 251–255.
- Fajaroh, F.; Setyawan, H.; Nur, A.; Lenggoro, I. W. Adv. Powder Technol.2013, 24 (2), 507–511.
CrossRef - Nuryono, N.; Syukur, M.; Kuncaka, A.; Sakti, S. C. W. Indones. J. Chem.2016, 16 (2), 130–137.
- Ursachi, I.; Vasile, A.; Chiriac, H.; Postolache, P.; Stancu, A. Mater. Res. Bull.2011, 46 (12), 2468–2473.
CrossRef - Wu, W.; He, Q.; Jiang, C. Nanoscale Res. Lett.2008, 3 (11), 397–415.
CrossRef - Roto, R.; Yusran, Y.; Kuncaka, A. Appl. Surf. Sci.2016, 377, 30–36.
CrossRef - Persson, J.A., Wennerholm, M., OHalloran, S. Handbook for; FOSS, DK-3400 Hilleroed: Denmark, 2008.
- Zhuravlev, L. T. Colloids Surfaces A Physicochem. Eng. Asp.2000, 173 (1–3), 1–38.
CrossRef - Shajesh, P.; Smitha, S.; Aravind, P. R.; Warrier, K. G. K. J. Sol-Gel Sci. Technol.2009, 50 (3), 353–358.
CrossRef - El-Naggar, A. Y. J. Emerg. Trends Eng. Appl. Sci.2013, 4 (1), 144–148.
- Sun, Z. H.; Xu, D.; Wang, X. Q.; Zhang, G. H.; Yu, G.; Zhu, L. Y.; Fan, H. L. Cryst. Res. Technol2007, 42, 812–816.
CrossRef - Si, S.; Kotal, A.; Mandal, T. K.; Giri, S.; Nakamura, H.; Kohara, T. Chem. Mater2004, 16 (11), 3489–3496.
CrossRef - Wang, Z.; Zhu, H.; Wang, X.; Yang, F.; Yang, X. Nanotechnology2009, 20 (46), 465606.
CrossRef - Kalska-Szostko, B.; Wykowska, U.; Satula, D.; Nordblad, P. Beilstein J. Nanotechnol.2015, 6, 1385–1396.
CrossRef - Pacławski, K.; Fitzner, K. Metall. Mater. Trans. B2004, 35 (December), 1071–1085.
CrossRef - Buhani; Narsito; Nuryono; Kunarti, E. S. Desalination2010, 251 (1–3), 83–89.
- Adamson W. A., Gast, P. A. Physical Chemistry of Surface, Sixth.; John Wiley & Sons: New York, 1997.
- Gurung, M.; Adhikari, B. B.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. Chem. Eng. J.2011, 174 (2–3), 556–563
CrossRef - Ho, Y. S.; Mckay, G. Process Biochem.1999, 34, 451–465.
CrossRef - Ramesh, A.; Hasegawa, H.; Sugimoto, W.; Maki, T.; Ueda, K. Bioresour. Technol.2008, 99 (9), 3801–3809.
CrossRef - Liu, L.; Liu, S.; Zhang, Q.; Li, C.; Bao, C.; Liu, X.; Xiao, P. J. Chem. Eng. Data2013, 58 (2), 209–216.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









