Preliminary Study on Natural Radionuclide Concentration in Samosir Area
Harlem Marpaung1, Jamahir Gultom1, Zul Alfian1 and Monica Gabryella1
Department of Chemistry, Sumatera Utara University, Medan, Indonesia.
Corresponding Author E-mail: harlemmarpaung@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330157
Article Received on :
Article Accepted on :
Article Published : 07 Feb 2017
Measurement of natural radionuclides concentration in Samosirarea of Sumatera island of Indonesia aims to obtain basis data of the content of radionuclides that could be tended in the development of the area. This research was conducted using Neutron Activation Analysis (NAA), which consists of a sampling of soil of SamosirIsland as the residential area and the soil surrounding ofPangururan hot-spring as the recreational area which then samples and standards were prepared. Then irradiated with thermal neutron flux 3.51013 n.cm-2.s-1 for 1 hour in a nuclear reactor, and then counted using a gamma spectrometer by HPGe detector. The analysis showed that the soil of Samosir Island samples with concentrations of Uranium of (<0.67) ppm and Thorium of (18.00 ± 0.49) ppm and soil surroundingof hot-spring Pangururan detected and found Uranium of (16.83 ± 0. 83) ppm and Thorium of (6.49 ± 0.35) ppm.
KEYWORDS:NAA; Natural radionuclide; Uranium and Thorium; Samosir Island
Download this article as:| Copy the following to cite this article: Marpaung H, Gultom J, Alfian Z, Gabryella M. Preliminary Study On Natural Radionuclide Concentration In Samosir Area. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Marpaung H, Gultom J, Alfian Z, Gabryella M. Preliminary Study On Natural Radionuclide Concentration In Samosir Area . Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=29871 |
Introduction
Radionuclides in the soil come from radioactive dust fall-out, the outcome of weathering of rocks containing natural radionuclides or derived from material from the eruption of the volcano (Artinigsih et al, 2008). Radionuclide is atom nucleus with unstable nucleus characterized by excess energy so it will emit radiation; alpha radiation, beta and gamma (Barnes, 1983). Naturally occurring radionuclides are also present in airborne particles as they are also present in soil particles able to be eroded, re-suspended or transported by the processes previously described, and also due to the radon exhalation from soil, which is especially significant to 210Pb and 210Po. The dust generated in the manipulation of NORM materials can be another pathway to increase naturally occurring radionuclide content in aerosol particles, although in some cases its contribution to the effective dose was assessed to be negligible, such as the use of phosphogypsum as soil amendment (Abril et al, 2009).
The present of certain number of natural radionuclides in the soil can lead to a danger, whereas soil provides useful resources for the survival of humans and other living creatures. The danger of radionuclides is external or internal radiation. Effect of external radiation is gamma radiation emitted from each radionuclide, while effect ofinternal radiation is the inhalation of radon and thoron which is gas natural radioactive which product of uranium and thorium decay that affect the incidence of lung cancer (Rasito, et al., 2008). Other U-238 decay is Ra-226 is an alpha emitter with a half-life of 1600 year. The origin of this radiation is due to Ra-226 and its decay products, which have been brought up to the earth’s surface by the water of hot springs. It enters the body through different paths such as ingestion of food, water or inhalation and replaces calcium and causes cancers and other body disorders due to a long half-life (Aguado et al., 2008; Ghiassi-Nejad et al,2003; Ushida and Tagami, 2009).
Natural radionuclide can be found in soil (Akpan et al, 2016). They did ground investigations of the activity concentrations from primordial radionuclides (238U, 232Th and 40K) in Akpabuyo, Southeastern Nigeria and the result showed the activity concentrations of the radionuclides of 238U, 232Th, and 40K are vary from 2.22 to 116.09 Bq kg-1 (with a mean value of 34.67 Bq kg-1), 3.65e87.41 Bq kg-1 (mean of 38.59 Bq kg-1) and 6.26e384.99 Bq kg-1 (mean of 114.66 Bq kg-1), respectively. Nguelem, et al (2016) reported a research to show activity concentrations in twenty five (25) soil samples collected from various points in bauxite ore deposit in Menoua Division in Western of Cameroon. The measurements of 40K, 226Ra, 232Th, 235U and 238U shown activity of 671 ± 272, 125 ± 58, 157 ± 67, 6 ± 3 and 99 ± 69 Bq kg−1, respectively.
Only a very few studies on radionuclide concentration in Indonesian mountain areas have been found in literature. Sri Artinigsih and Wijiyono (2008) performed a study on the concentration of radionuclides in the soils around the mount of Merapi in Central of Java island of Indonesia. The study conducted after the eruption of the mount Merapi. The concentrations of K-40, Zn-65, Br-82, Zr-97, Sb-121, Ice-137, Bi-211, Pb-212, Bi-214, Pb-214, Sc-216, Th-222, Sb-224, Ra-226, Ac-228, and U-235 were measured. The results showed that natural radionuclides (Ra-226, K-40, Th-222 and U-235) were found in the mount area. It was stated that those natural radionuclides come from lava bursts due to the activities of mount Merapi eruption last date which is usually followed by bursts of material, dust and some rocks. In the past, about 73,000 ± 4,000 years BCE, there was a big eruption of an ancient volcano (Mount Toba) named as Toba eruption. The eruption yielded an island in the middle of its top, known as SamosirIsland. This island resulted by the pressure of magma that has not come out of the eruption (Pardosi, 2015). This fact reveals that it is possible that the Samosir Island which was the resulted from a volcanic eruption also contain natural radionuclides.
In Northern and Central Sumatra island of Indonesia a wide distribution of ignimbrite and volcanic sediments have been observed.Some geologists considered that these volcanic products were erupted from Lake Toba (Nishimura, et al. 1983). In volcanic areas, several hot-springs are usually found. Water of the hot-spring are also found in the areas of Samosir island, one of the hot-spring is Pangururan hot-spring. Water of the hot-spring come from ground-water discharge in the earth’s crust after being heated by geothermal and usually temperatures around ≥37°C (Meinzer, 2002). Because of Samosir Island resulted of a volcanic eruption, the soil of Samosir Island may contain natural radionuclides and also in the soil surrounding of Pangururan hot-pring. And the elements contained in the hot water distributed in the surrounding soil. To the best knowledge of the authors, the research on the natural radionuclides in the soil surrounding of hot-spring of the Samosir Island has not found in literature.
This study focuses on determination of concentration and distribution of naturally occurring radionuclides in Samosir island of Sumatera Utara province of Indonesia. The objective is to measure the concentration of natural radionuclides in the areas which suffered from ancient eruption. The result of this study is expected to supply the necessary information for the government of Indonesia.
Materials and Methods.
Sampling and Sample Preparation
Soil of Samosir Island samples were taken from three different locations soil of Samosir Island, which were TanoPonggol, Kantor BupatiRianiate and PasirPutihParbaba. And soil surrounding of Pangururan hot-spring was taken ± 30 cm from hot-spring pond. Samples were taken in the depth of 5-10cm from the ground. Soil samples then inserted in polyethylene plastic and sealed.Soil samples were dried byroom’s temperature. After drying, the samples were crushed and sieved to 200 mesh sizes and then inserted in a polyethylene plastic and sealed.
Washing Polyethylene Vial for Sample Sites
Polyethylene vial inserted into a beaker containing nitric acid (HNO3 50%), then shaken for 2-3 minutes and soaked for 24 hours. And then drybyroom’s temperature for 15 minutes. After drying, the vial was inserted into a beaker and sealed to avoid contamination
Irradiation Samples and Counting
Irradiation Soil of Samosir Island Sample
Weighed 0.034740 g of soil of Samosir Island sample and weighed 0.039368 g of standard. Then, inserted each into vials polyethylene (LDPE) and wrapped with aluminum foil. Irradiated withthe power is 15MW and thermal neutron flux 3,5.1013 n.cm-2.s-1for 1 hour. After irradiation, the samples were allowed to stand for 3-4 weeks.
Irradiation Soil Surrounding of Pangururan Hot–Spring Sample
Weighed 0,039927g of soil surrounding of Pangururan hot-spring sample and weighed 0,037491 g of standard. Then, inserted each into vials polyethylene (LDPE) and wrapped with aluminum foil. Irradiated with the power is 15MW andthermal neutron flux 3,5.1013 n.cm-2.s-1for 1 hour. After irradiation, the samples were allowed to stand for 3-4 weeks.
Counted Using Gamma Spectrometer
Aluminum foil released from each capsules by using tweezers. Then put the activated capsules into HPGe Gamma Spectrometer and closed HPGe detector. Then, counted for 1 hour.
Data Analysis
Qualitative Analysis
The qualitative analysis is based on the characteristics of gamma ray energy for each radionuclides in the sample compared to the energy spectrum of gamma isotope table.
QuantitativeAnalysis
The quantitative analysis was done by 2 methods, there are the comparative method (comparison) and ko non-comparative method. The comparative method is conducted by comparing the count of sample with count of standard that concentration of analyzed elements. Meanwhile, ko non-comparative method be calculated directly by using ko –NAAsoftware.
Results And Discussion.
Calibration Curve
By using Ba-133, Cs-137 and Co-60 reference source of gamma Ba-133, Cs-137 and Co-60 calibration data obtained for energy as seen in Table 1, where the number of stripes (Channel) as Xi and energy (keV) as Yi. Determination of the energy curve will affect the quantity of elements in the sample and standard-setting.
Table 1: Data calibration by using standards Ba-133, cs-137 and co-60 source energy
| No. | Xi | Yi |
| 1 | 214 | 81 |
| 2 | 927 | 356,8 |
| 3 | 1719 | 661,66 |
| 4 | 3044 | 1173,5 |
| 5 | 3457 | 1332,24 |
By using the data of table 1 obtained a calibration curve energy in the form of linear line made bychannel and energy which visible on the monitor screen MCA. As for the energy calibration curve can be seen in Figure 1.
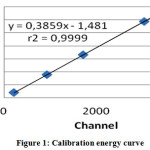 |
Figure 1: Calibration energy curve |
Qualitative Analysis
Gamma Energy Soectrum of soil of Samosir Island sample can be seen inFigure 2.
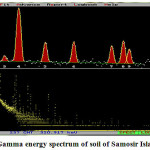 |
Figure 2: Gamma energy spectrum of soil of Samosir Island sample |
And isotope table of Uranium and Thorium can be seen in Table 2.
Table 2 : Isotope table of uranium and thorium
|
Energy (keV) |
Element | Nuclide was Formed | Half-Time (T1/2) |
| 312,01 | Th | Pa-233 | 27.0 days |
| 277,60 | U | Np-239 | 2,36 days |
Quantitative Determination
Thorium Concentration Determination
Thorium Concentration Determination was done by the comparat
ive method (comparison) conducted by the equation (Mulyaningsih, 2002):
![]()
Where, W is weight. Thus, weight of Thorium in soil of Samosir Island sample is:
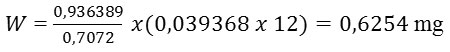
With the result that concentration of Thorium in ppm (mg/kg) is:
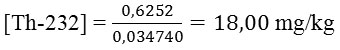
Analysis Uncertainty using the equation (Mulyaningsih, 2002) :
U = Uc.k (2)
Uncertainty = concentration of element x U (3)
Where U is the uncertainty stretch (%), Uc is the combined uncertainty (%) and k is the coverage factor and confidence level of 95%. So we get the uncertainty thorium concentration in soil ofSamosir Island sample is:
U = 1,38098244 x 1,96 = 2,706725583 %
Uncertainty = 18,00 x 2,706725583 % = 0,49
Thus, from the above calculations obtained concentration of Th-232 in the soil of Samosir Island is 18.00 ± 0.49 mg / kg.
And, concentration of Thorium in soil surrounding of Pangururan hot-spring sample obtained:
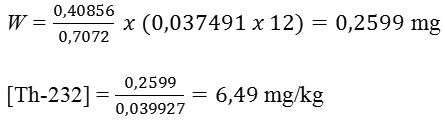
By uncertainty,
U = 2,747587 x 1,96 = 5,385271475 %
Uncertainty: 6,49 x 5,385271475 % = 0,35
With the result, concentration of Thorium in soil surrounding of Pangururan hot-spring amounted 6,49±0,35 mg/kg.
Uranium Concentration Determination
Uranium quantitative determination was conducted by ko non-comparative method. This is because the energy of formation of Uranium (277.60 KeV) only detected in sample, but not in the SRM (Standard Reference Material), so it does not do a comparison between the count data of sample with count data of SRM. The principal parameters of ko-NAA method has been defined and is already available in ko-NAA software, so by measuring the amount of counted samples and parameters of the reactor, the concentration of elements in the sample can be calculated directly by ko-NAA software. By the equation (Corte and Simonits, 1994):
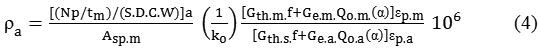
Where, parameters in this equations namely, ρa is the concentration of analyte elements (mg/kg or mg/g); Npis the amount of count is collected at the height of the energy-filled after correction of the missing pulse (the dead time detector and the effect of the coincidence); S is the saturation factor is expressed as S = 1-e λ.tirr, λ = decay constancy, λ = (ln 2)/T, with T = radionuclide half-time, tirr = irradiation time (second); Dis decay factor = e -λ.td, td = decay time; Cis measurement factor = (1-e-λ.tm)/ λ.tm , tm = measurement time (second);Wis mass of irradiated element (g or kg);εpis detection efficiency of energy peaks intact including correction for attenuation γ; ASPis a specific count rate, Gth is self-absorption correction factor for thermal neutrons; Geis the self-absorption correction factor for epithermal neutrons; α is parameter for the distribution of neutron flux; φe’ ˷ 1/E1+α, f is the ratio of the thermal flux of the epithermal; Qα(α) is the ratio between the integral resonance in the cross section of thermal neutrons, and index of a,m are each claiming the analyte and the observe monitor flux;
Factors of ko involves the principal parameters which can be expressed by equation 5 below:

In equation 5, parameters are M= mass number of radioisotope; γ= the fraction of gamma energy emitted by a radioisotope; θ= abundance of isotope in nature; σ= cross section of the thermal neutron absorption in the reaction (n, γ).
Therefore, the concentration of uranium in soil of Samosir Island of <0.67 mg/kg, and the concentration of uranium in the soil surrounding of Pangururan hot-springof 16.83 ± 0.83 mg/kg.
Conclusions
From the research that has been done obtained the results as:
The analysis showed that the soil of Samosir Island samples with concentrations of Uranium of (<0.67) ppm and Thorium of (18.00 ± 0.49) ppm and soil surrounding of hot-spring Pangururan_Samosir detected and found Uranium of (16.83 ± 0. 83) ppm and Thorium of (6.49 ± 0.35) ppm
To determine the impact of Thorium and Uranium necessary to measure the concentration of activity emitted by Thorium and Uranium.
Acknowledgement
The author would like to thank Ms. Th.Rinawho helping us to conduct this research at BATAN (National Atomic Energy Agency of Indonesia).
References
- Abril, J.M., García-Tenorio, R., Peri_a~nez, R., Enamorado, S.M., Andreu, L., Delgado, A.,. Occupational dosimetric assessment (inhalation pathway) from the application of phosphogypsum in agriculture in south west Spain. J. Environ. Radioact.2009,100, 29-34.
CrossRef - Aguado, J.L., Bolivar, J.P., Garcia-Tenorio, R., 226Ra and 228Ra determination in environmental samples by alpha- Particles spectrometry. J. Radioanal. Nucl. Chem. 2008.278 (1), 191-199.
- Akpan, A., Paul, N., Uwah, E. Ground radiometric investigation of natural radiation levels and their radiological effects in Akpabuyo, Nigeria. journal of African Earth Sciences].Department of Physics, University of Calabar, Calabar, Nigeria2016.
- Artiningsih, S., Wijiyono..IdentifikasiRadionuklidaPemancar Gamma padaSampel Abu Vulkanik, Sedimen, Tanah danPasir di SekitarGunungMerapiPascaErupsi2006 [Prosiding Seminar]. Yogyakarta: PusatTeknologiAkseleratordan Proses Bahan – BATAN.20
- Barnes. P. Structure and Performance of Cements. Applied Science Publishers. London 1983.
- Corte, D and Simonits, F. Vade Mecum for ko–Users. DSM Research. Geleen.1994.
- Ghiassi-Nejad, M., Beitollahi, M.M., Asefi, M., Rezanejad, F.,. Exposure to 226Ra from consumption of vegetables in the high level natural radiation area of Ramsar- Iran. J.Environ. Radioact.2003., 66, 215-225.
CrossRef - Gunandjar..Analisa Uranium dan Thorium dalamLimbahRadioaktifdari Proses DaurBahanBakarNuklir. PusatTeknologiLimbahRadioaktif – .BATAN.197
- Meinzer, O.E..IlmuPengetahuanBumidanEnergi. Jilid 3. Jakarta: PT. Widyadara.,2002
- Mulyaningsih, R. 15 Oktober. AnalisisSampelGeologidenganMetodeAnalisisAktivasi Neutron di RSG-GAS. [Prosiding Seminar Nasional ke-8 TeknologidanKeselamatan PLTN SerloFasilitasNuklir]. PusatPengembanganTeknologiReaktorRiset (P2TRR) –BATAN2002
- Mulyaningsih, R. PenaksiranNilaiKetidakpastianPengukuranPadaPenentuan Scandium dalam Marine Sediment denganMetodeAnalisisAktivasi Neutron. [JurnalTeknologiReaktorNuklir]. BATAN.,2005.
- Nishimura, S. Abe, E. Nishida, J. Yokoyama, T. Dharma, A. Hehanussa, P. Hehuwat, F. A Gravity and Volcanostratigraphic Interpretation of The Lake Toba Region, North Sumatra, Indonesia. [Journal]. Elsevier Science Publisher B.V. Amsterdam1983.
- Nguelem, E., Ndontchueng, M., Motapon, O., Determination of 226Ra, 232Th, 40K, 235U and 238U activity concentration and public dose assessment in soil samples from bauxite core deposits in Western Cameroon. [journal]. Department of Physics, The University of Douala, Douala, Cameroon.,2016.
- Pardosi, A. Supervolcano Toba. Balige: DinasKebudayaandanPariwisataKabupaten Toba Samosir.,2015.
- Rasito. Zulfakhri. Arianta, P.A. Suherman, A..Konsentrasi Uranium, Thorium danKaliumdalamBerbagaiProduk Semen yang Dipasarkan di Indonesia. [jurnal]. JurusanFisika FMIPA UniversitasUdayana – Bali PusatTeknologiNuklirBahandanRadiometri, BATAN – Bandung.,2008
- Ushida, S., Tagami, K., Transfer of radium-226 from soil to rice: a comparison of sampling area differences. J. Nucl. Sci. Technol. 2009.46 (1), 49e54.

This work is licensed under a Creative Commons Attribution 4.0 International License.









