Phenolics and Ascorbic Acid Related to Antioxidant Activity of MaoFruit Juice and Their Thermal Stability Study (Review Article)
Thitiya Sripakdee, Ratana Mahachai and Saksit Chanthai
Materials Chemistry Research Center, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand.
Corresponding author E-mail: sakcha2@kku.ac.th
DOI : http://dx.doi.org/10.13005/ojc/330108
Article Received on :
Article Accepted on :
Article Published : 17 Feb 2017
Antioxidantand/or anti-aging activities are always linedwith people’s minds as major potential benefits concerning human health in the recent commercial features for an economicworld of foodstuffs and medical uses. Total phenolics includingflavonoids and anthocyanins,and ascorbic acid in the Mao juices areclosely related to their antioxidant activity.Numerous research approaches on these functional foods, in particular the colored fruits and vegetableshave been investigated. Method validation and determination ofthe potential compounds have been increasingly developedwith highly sensitive and selective procedures and applications including thermal stability of the Mao juice.Their antioxidant activities obtained from different assays related to the contents of both phenolics and ascorbic acid in the anthocyanins-richMao juicesin Thailand are reported and discussed.
KEYWORDS:Antioxidant activity; Mao juice; Phenolic compounds; Flavonoids; Anthocyanins; Ascorbic acid; Thermal stability
Download this article as:| Copy the following to cite this article: Sripakdee T, Mahachai R, Chanthai S. Phenolics and Ascorbic Acid Related to Antioxidant Activity of MaoFruit Juice and Their Thermal Stability Study (Review Article). Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Sripakdee T, Mahachai R, Chanthai S. Phenolics and Ascorbic Acid Related to Antioxidant Activity of MaoFruit Juice and Their Thermal Stability Study (Review Article). Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=30311 |
Introduction
Nowadays, there is growing interest in useof polyphenolic antioxidant-rich plants as dietarysupplements1,2.Health expertsrecommendto increase the consumption of fruit since there is an evidence linking a diet rich in fruit withreduced incidence of various age relating chronic diseases3.The protective effect of fruits has beenattributed to their bioactive antioxidant constituents,including polyphenols and vitamins4.Polyphenols are secondarymetabolites presenting in all plant tissues. They are important antioxidantsof human diet5.Generally, vitamin C or ascorbicacid is also majorreducing agent of the essential phytonutrients forthe metabolism of living cells. Both polyphenols and ascorbic acidare the two major contributors to the total antioxidant activityin vegetable and fruit. It was reported that vitaminC accounted for 65-100%of antioxidant capacity ofcitrus juices6. In addition, some fruitshad very high level of total phenolic content and reducingactivity7.
Mao tree(Antidesma thwaitesianum Muell. Arg.)is classifiedin the family Euphobiaceae. It isone of many tropical compiled fruits like wild berry which have been known for longtime in Thailand, especially in Phu Phan district, Sakon Nakhonprovince. There are commonly consumed as commercially availableproducts ofjuice and wine, particularly thefruit juice contains a very rich source of antioxidants. The recent studies have investigated that the healthbenefits and antioxidant activities of Mao Luang fruits aremainly attributed to almost known phenolic compounds including anthocyanins, proanthocyanidins andtannins, resveratrol and benzoic, caffeic and cinnamic acids4,8–10.Total phenolics, antioxidant activity and nutritivevalues of the Mao fruits have previously been reported. Polyphenolic compounds and proanthocyanidinsisolated from the fruit extract exhibited much higherantioxidant activities than that of standard Troloxand had similar antioxidant potential to grape seed proanthocyanidin extract11.Fifteen varieties of Mao Luang fruitscontained three different flavonoids (catechin,procyanidin B1 and procyanidin B2)9.These organic compounds are the majorflavonoids in all analyzed fruit samples. The skincontact the Mao Luang red wine had higher amountsof flavonoids, phenolic acids, anthocyanins, organicacids than the non-skin contact one4.Since the great importance of antioxidant activityis mainly attributed from polyphenols and ascorbic acidfor human health and also because of the growth incommercial fruit juice production and consumption3.The part of flavonoids has manybeneficial effects on human and animal health. These are the best known natural antioxidants12-15.
Therefore,this review wasaimed to describe total phenolics and ascorbic acid in association with theirantioxidant activity and thermal stabilityof the Mao fruit juices.Theirantioxidant activity was comparatively assayed by some antioxidant methods including1,1-diphenyl-2-picrylhydrazyl(DPPH), 2,2´-azinobis-3-ethylbenzothiazoline-6-sulphonate (ABTS), N,N-dimethyl-p-phenylenediamine dihydrochloride (DMPD)and ferric reducing antioxidant power (FRAP). The heating effect on total phenolics of the Mao juice was also investigated. Determination of antioxidant activity, total phenolics, and flavonoids and their correlationstudies among total phenolics and flavonoidcontents versus antioxidant activity in the fruit juice fromvarious production sources were investigated. Moreover, total anthocyanin was evaluated by using sample dilution method in association with an external calibration curve under optimum conditions by UV-Visible and fluorescence spectrophotometry comparing with pH-differential method16.
The Mao Fruits
The so-called Maofruit is classified as a kind of wild berry fruits. There are about eighteen species of Antidesma plants. Each fruit is an oval shape with dark green, turns to orange-red and dark-purple at a fully ripe stage and becomes sweet with slightly tart10. Theripe fruit is favorable to be consumed and sold in thelocal market because of its good color and taste. BothMao juice andits wine have become more popularas healthy nourishment 10.At present, the health effects of the Mao fruit have been interested. Various publications have been reported as follows.
Organic acids in ripe fruits of fifteen cultivars of Mao Luang from Phuphan valley, Sakon Nakhon were determined. The results showed that there were two groups of organic acids contents in ripe fruit of Mao Luangcultivars. The major group includes tartaric acid, ascorbic acid, citric acid and benzoic acid, and the minor one includes malic acid, lactic acid, oxalic acid and acetic acid4. Changesin physico-chemical properties, polyphenolics and antiradical activityin Mao Luang fruit during developmentand ripening were investigated. At over ripe stage, the fruits had the highest antioxidants. This data attribute a good basis forevaluating the nutritional importance of the Mao fruit8. Flavonoids in these ripe fruits also from the Phuphan valleywere also determinedby RP-HPLC9.
Polyphenolic contents in both Mao seeds (MS) and Mao marcs (MM) were purified and investigated their radicalscavenging activities against DPPH and ABTS radicals and thiobarbituric acid reactive products (TBARP)10–14. The resultsshowedboth MS and MM were an abundant source of polyphenols andproanthocyanidins. Their radical scavenging activities of MS and MM against DPPH and ABTS radicals were significantly higher than that of standard Trolox. In addition, theantihypertensive and antioxidative effects of Mao pomace (MP) using hypertensive rats. The MP treatment significantly prevented the increase in blood pressure,hind limb blood flow and vascular resistance of L-NAME treated hypertensive rats15,16. The present results provide an evidence for theantihypertensive effect of MP and suggest that MP might be useful as a dietary supplementagainst hypertension.
Evaluation of the antioxidant activityin Thai fruit wines was done using both off-line DPPH assay and on-line HPLC–MS–DPPH assay for the analysis of phenolic antioxidantcompounds17. An LC–MS/MS was very usefulto quantify the active compounds.Thisresult shows that the on-line HPLC–MS–DPPH assay can be powerful for rapid characterization of antioxidant compounds in plant extracts.The cytotoxic and antioxidant activities of the fruit and fruit waste (residue andmarc)extracts of Maowere also studied using chemical andcell-based assays18. The results showed that the ethanol extracts of fresh and dried Mao fruits exhibit both cytotoxic and cellularantioxidant activities, and thus possess great potentials for application in the development of effectivedietary supplements to prevent oxidative stress-induced diseases.
Chemistry Background of Mao Juice
The chemistry background of the Mao juice including pH, total acidity, volatile acidity, fixed acidity, total solids, specific gravity and buffering capacity was determined following the standard AOAC methods16.Total acidity ofthe fruit juice was determined by titration method. The solution of dilute juice was titrated to pH 8.1 with0.1 M NaOH standard solution using phenolphthalein. Total acidity was calculated in termsof citric acid using its chemical formula: Acidity (g/100 mL) = (Normality of the juice sample) x (Equivalent weight of citric acid). The pH value of10% diluted juice was also determined using pHmeter.
Volatile acidity was also determined by titrationmethod. The solution of the residual juice sampleafter heat treatment was titrated with 0.1 M NaOHstandard solution until the end point reached at pH8.1 by pH meter. Fixed acidity is simplythe difference between totalacidity andits volatile acidity. It refers to the amount of aciditythat is not volatile under normal conditions. The fixedacidity was calculated according to the equation: Fixed acidity (as citric acid) = (Total acidity (g/L) as citric acid) – (Volatile acidity, g/100 mL as citricacid x 10 x 1.17).
Buffering capacity is the ability of typical buffersolution to resist changes in pH. The initial pH wasmeasured with pH meter and the buffering capacitywas measured by adding 1 M NaOH in the incrementsof 0.2 mL until the pH value reached at pH 9.0. Buffering capacity is expressed as the molarity ofsodium hydroxide required to increase pH value by1.0 unit19.Total solids are a measure of the amount ofmaterials remaining after all the water has beenevaporated. It is needed to clarify all dissolvedmaterials including both inorganic (salts) and organiccompounds presence in the juice sample. Five mL ofthe fruit juice was dried in hot oven at 70oC for 24 h. The residual weight was accurately obtained and thencalculated as percentage of the sample used.
Free Radicals and Antioxidants Activity
Free radicals are highly reactive chemicals that have the potential to harm cells. They are created when an atom or a molecule (a chemical that has two or more atoms) either gains or loses an electron (a small negatively charged particle found inatoms)20,21.Therefore, some may be behaving as oxidants or reductants22.The most important oxygen-containing free radicals in many disease states are hydroxyl radical (•OH), oxygen singlet (1O2), superoxide anion radical (O2•-), hydrogen peroxide (H2O2), peroxyl (ROO•), hypochlorite (OCl–), nitric oxide radical (NO), and peroxynitrite radical (OONO–)23. The built-up of free radicals over time may contribute to the aging process and the development of health conditions such as cancer, heart disease, atherosclerosis, Alzheimer’s disease, inflammation and diabetesand24.
Antioxidants are molecule that inhibits the oxidation of other molecules. They are interacted with and neutralize free radicals, thus preventing them from causing damage. Antioxidants are also known as “free radical scavengers’19,20. They have been traditionally dividedinto two classes; primaryor chain-breaking antioxidants, and secondary or preventative antioxidants.
Primary antioxidants orchain-breakingantioxidants, AH, when presentin trace amounts, may either delay or inhibit the initiation stepby reacting with a lipid radical or inhibit the propagation step by reacting with peroxyl or alkoxyl radicals25–27.
Chain-breaking mechanisms:
L• + AH → LH + A•
LO• + AH → LOH + A•
LOO• + AH → LOOH + A•
The antioxidant free radical may further interfere with chainpropagation reactions by forming peroxy antioxidant compounds:
A• + LOO• → LOOA
A• + LO• → LOA
The activation energy of the above reactions increases withincreasing A–H and L–H bond dissociation energy. Therefore,the efficiency of the antioxidant increases with decreasing A–Hbond strength27–30.A classification of antioxidant assays is defined as the type of reaction:hydrogen atom transfer (HAT)-based assaysand electron transfer (ET) – based assays
HAT-based assays are used to measure the capability of an antioxidant to quench free radicals by H-atom donation. The HAT mechanisms ofantioxidant action in which the hydrogen atom (H) of a phenol (Ar–OH) is transferred to a ROO• radicalas summarized by the reaction:
ROO• + AH/ArOH → ROOH + A•/ArO•
where, the aryloxy radical (ArO•) formed from the reaction of antioxidant phenol with peroxyl radicalthat is stabilized by resonance. Effective phenolic antioxidants need to react faster than biomolecules withfree radicals to protect the later from oxidation. The HAT-based assays include oxygen radical absorbance capacity (ORAC) assay, TRAP assay usingR-phycoerythrin as the fluorescent probe, crocin bleaching assay using 2,2′-azobis(2-amidinopropane)hydrochloride (AAPH) as the radical generator, and β-carotene bleaching assay31–32
ET-based assays(electron transfer) are used to measure the capacity of an antioxidant in the reduction of an oxidant, which changes in color when reduced. The degree of color change is correlated with the sample’s antioxidant concentrations. ET-based assays include the total phenols assay by Folin-Ciocalteu reagent (FCR), Trolox equivalence antioxidant capacity (TEAC), ferric ion reducing antioxidant power (FRAP), total antioxidant potential assay using a Cu(II) complex as an oxidant, and DPPH. In addition, other assays are used to measure a sample’s scavenging capacity of biologically relevant oxidants such as singlet oxygen, superoxide anion, peroxynitrite, and hydroxyl radical31–32. The reactionequations of various ET-based assays can be summarized as follows:
Folin: Mo(VI) (yellow) + e– (from AH) → Mo(V) (blue)
where, the oxidising reagent is a molybdophosphotungstic heteropolyacid comprised of3H2O–P2O5–13WO3–5MoO3–10H2O (heteropoly anion: P2Mo5W13O626–), in which the hypothesizedactive center is Mo(VI) with λmax 765 nm.
FRAP: Fe(TPTZ)23+ + ArOH→ Fe(TPTZ)22+ + ArO• + H+
where, TPTZ: 2,4,6-tripyridyl-s-triazine ligand with λmax 595 nm.
Ferricyanide/Prussian blue:
Fe(CN)63– + ArOH → Fe(CN)64– + ArO• + H+
Fe(CN)64– + Fe 3++ K+ → KFe[Fe(CN)6]
where, KFe[Fe(CN)6]: Prussian blue with λmax 700 nm.
ABTS/TEAC: ABTS + K2S2O8 → ABTS•+
ABTS•+ + ArOH → ABTS + ArO• + H+
where, ABTS is 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) with λmax 734 nm and TEACis Trolox-equivalent antioxidant capacity (also the name of the assay). Although other wavelengths suchas 415 and 645 nm have been used in the ABTS assay, the 734 nm peak wavelength has been predominantlypreferred due to less interference from plant pigments.
DPPH: DPPH• + ArOH→ DPPH + ArO• + H+
where, DPPH• is the [2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl] stable radical with λmax 515 nm.
CUPRAC: nCu(Nc)22+ + Ar(OH)n → nCu(Nc)2+ + Ar(=O)n+ nH+
where, the polyphenol with suitably situated Ar–OH groups is oxidized to the corresponding quinone,shows absorption maximumat 450 nm 32–35.
Kinetically antioxidants can be classified into six categories as follows:
Antioxidants that break chains by reacting with peroxyl radicals having weak O-H or N-H bonds such as phenol, napthol, hydroquinone, aromatic amines and aminophenols.
Antioxidants that break chains by reacting with alkyl radicals such as quinones, nitrones, iminoquinones.
Hydroperoxide decomposing antioxidants such as sulphide, phosphide, thiophosphate.
Metal deactivating antioxidants: diamines, hydroxyl acids and bifunctional compounds.
Cyclic chain termination by antioxidants: aromatic amines, nitroxyl radical, variable valence metal compounds.
Synergism of action of several antioxidants: phenol sulphide in which phenolic group reacts with peroxyl radical and sulphide group with hydroperoxide 36.
Screening Testing for Antioxidant Activity
DPPH free Radical Scavenging Assay
The DPPH assay is based on the reduction of DPPH, a stable free radicalwith an unpaired electron that is delocalized over the entire molecule. The DPPH radical has been used widely in themodel system to investigate the scavenging activities ofseveral natural compounds such as phenolic compounds,anthocyanins, or crude extracts of plants37.The free radical DPPH is scavenged by antioxidants through the donationof hydrogen, forming the reduced DPPH-H•. The colorchanged from purple to yellow after reduction, which canbe quantified by its decrease of absorbance at517 nm38,39
Measurements are made using a UV-visible spectrophotometerat room temperature, and the scavenging capacity is represented as the percentage of DPPH radicalinhibition. The DPPH assay is based on both electron transfer (ET) and hydrogen atom transfer (HAT) reactions32.An advantage of the DPPH assay is that it is an easy, rapidand commercialmethod to evaluate the radical scavenging activity of non-enzymatic antioxidants16. Since DPPH isa stable radical, this assay considers not only the concentration of the tested sample, but also thereaction time and the temperature; both of which when controlled carefully enable this assay to behighly reproducible39.Antioxidant activity is expressed as BHT equivalent.
DMPD Radical Scavenging Activity Assay
N,N-Dimethyl-p-phenylenediamine dihydrochloride (DMPD) is a compound that is generally used to measure the antioxidant potentials of fruit juice, vegetable and other natural products. This assay focuses on the ability ofthe antioxidant compounds (AOH) to transfer a hydrogen atom to the coloredradical DMPD.+turning it into an uncolored DMPD+ compound40. In the presence of ferriciron, it gets converting to DMPD.+radicals, which are scavenged byantioxidant molecules present in the test samples41. The maximum wavelength of DMPD.+ shows at 505 nm.
DMPD (uncolored) + oxidant (Fe3+) + H+ → DMPD.+ (purple)
DMPD.+ (purple) + AOH → DMPD+ (uncolored) + AO
The reaction is rapid and the end point, which is stable, is takenas a measure of the antioxidative efficiency. Antioxidant ability is expressed as TEAC (Troloxequivalent antioxidant capacity).
ABTS Radical Cation Decolorization Assay
The 2,2’-azino-bis(3-ethylbenzothiazolin-6-sulfonic acid (ABTS) assay is based on the abilityof antioxidant molecule to scavenge the stableABTS radical. In this assay, ABTS.+ radical cation is formed from the reaction between ABTS and potassium persulfate, which is intensely colored. The antioxidant activity is measured as the ability of antioxidant to decrease the color reacting directly with ABTS.+ radical cation and can be measured the absorbance at 734 nm. The results are expressed as Troloxequivalent antioxidantcapacity (TEAC). Due to ABTS.+ radical cation is soluble in both aqueous and organic solvents and is not affected by ionic strength, so it can be used in multiple media to determine both hydrophilic and lipophilic antioxidant activity of sample. Furthermore, it is simpler and cheaperand allowing the evaluation of its rate of consumption with minimal interferences. Therefore, the method has been applied to the measurement of thetotal antioxidant activity of solutions of pure substances, aqueous mixture of beverages and biological fluids42–46.
Ferric Reducing Antioxidant Power (FRAP) Assay
The ferric reducing antioxidant power (FRAP) assay is based on the ability of antioxidants to reduce Fe3+ to Fe2+ in the presence of tripyridyltriazine (TPTZ) forming an intense blue of the Fe2+-TPTZ complex with an absorbance maximum at 593 nm47. At low pH, ferric iron (Fe3+) is initially reduced by electron-donating antioxidants present within the sample to its ferrous form (Fe2+). As the ferric to ferrous ion reduction occurs rapidly with all reductants with half reaction reduction potentials above that of Fe3+/Fe2+, the values in the FRAP assay expresses the corresponding concentration of electron donating antioxidants48. Increasing absorbance indicates an increase in reductive ability49,50.
The FRAP assay actually measures onlythe reducing capability based upon the ferric ion, which is notrelevant to antioxidant activity mechanistically and physiologically. Often, FRAP valueshave a poor relationship to other antioxidant measures. However, in contrast to other tests of total antioxidantpower, the FRAP assay is simple, speedy, inexpensive, androbust and does not require specialized equipment. The FRAPassay can be performed using automated, semiautomatic, ormanual methods50–53
Phenolic and Polyphenolic Compounds
Polyphenols, a large class of chemicals which are found in plants, have attracted much attention in the last decades due to their properties and the hope that they will show beneficial health effects, when taken as a dietary input or as complement54. Polyphenols are polyhydroxylated phytochemicals,which have common structures. They are divided into severalclassesaccording to the number of phenol ringsthat they contain and to the structural elements that bind these rings to one another. The main groupof polyphenols isflavonoids,phenolic acids, and stilbenoids.
In the present, there is growing interest on plant-derived polyphenols because of their potentialantioxidant and antimicrobial properties. Phenolic compounds exhibit considerable free-radical scavenging activity, which is determined by their reactivity as hydrogen- or electron-donating agents, their reactivity with other antioxidants and their metal chelating properties, as well as the stability of the resulting antioxidant-derived radicals.Although determination of polyphenols is hampered by their structural complexity anddiversity, several methods have been used to determine polyphenols in sample, then spectrophotometry in the ultraviolet region may be a useful tool to help resolve thisproblem55.
Colorimetric reactions are widely used in the UV-Visible spectrophotometric method, which is easy toperform, rapid and applicable in routine laboratory use, and low cost56. Total phenolic contents are well determined by using Folin-Ciocalteu method with gallic acid as standard. This method is based on the reduction of phosphomolybdic-phosphotungstic acid (Folin) reagent to a blue-colored complex in analkaline solution occurs in the presence of phenoliccompounds. The maximum absorption of the chromophores dependson the alkaline solution and the concentration of phenolic compounds. The absorbance can be measuredat 765 nm and the results areexpressed as mg gallic acid equivalents.
Phenolic compound + [P2W18O627– + H2P2Mo18O626–] → Blue complex
Folin-Ciocalteu reagent; Yellow
Flavonoids
Flavonoids are characterized as the group of organic compounds containing two or more aromatic rings, two benzene rings (ring A andB) joined by a linear three-carbon chain. The centralthree-carbon chain may form a closed pyran ring (ring C) with one of the benzene rings. It contains one or more phenolic hydroxyl groups. Flavonoids are themselves divided into 6 subclasses,depending on the oxidation state of the centralpyran ring: flavonols, flavones, flavanones, isoflavones,anthocyanidins and flavanols.
Flavonoids have many beneficial effects on human and animal health such as anti-aging, antioxidant, antibacterial and antifungal activities, anticancer, anti-cardiovascular disease and anti-inflammatory13. The capacity of the phenolic compounds and flavonoids to act as antioxidants depends upon their position of hydroxyl groups and other features in the chemical structure which is important for their antioxidant and free radical scavenging activities14, 57,58.
Total flavonoids were determined using aluminiumchloride colorimetric method. This method is based on the formation of a complex flavonoid-aluminum. Firstly,aluminium chloride forms acid stable complexes with the C-4 keto group and either the C-3 or C-5 hydroxyl group of flavones and flavonols. In addition, it also forms acid labile complexes with the ortho-dihydroxyl groups in the A- or B-ring of flavonoids. The colored flavonoid-aluminium complex formationobtained can be detected at maximum wavelength of 510 nm. The flavonoid contents were calculated from the linear equation based on the calibration curve and expressed as mg of catechin equivalents59.
Anthocyanins
Anthocyanins/polyphenolics are the natural colorants responsible for the red, blue, and purple colors of many fruits, vegetables and plants. They have several biological activities, including antioxidant, anti-inflammatory, anti-tumor, neuroprotective, anti-diabetic and cancer chemopreventive agents57–68. Additionally, they are widely used as colorants in food industry 69. Anthocyanins are a subclass of flavonoids and represent one of the most widely distributed classes of flavonoids in various plants 70. They are glycosides of anthocyanidins, which vary with different hydroxyl or methoxyl substitutions at the 3 and 5 positions on the A and C rings in their basic flavylium structure 71,72. The difference in chemical structure and color of anthocyanins depends on several factors including pH, temperature, light intensity, amount of pigments, metallic ions, ascorbic acid and sugars (glucose, galactose, rhamnose, xylose and arabinose). Moreover, their variations by acylation of the sugar groups with acids occur. In acidic conditions, there are four anthocyanin structures present in equilibrium: flavyliumcation, quinonoidal base, carbinol pseudobase and chalcone71,73. Anthocyanins can commonly be found in numerous fruits, especially various kinds of berries, their juices as well as wines 74,75,76.
Evaluation of total anthocyanins in fruits and other plants are usually relied on spectrophotometric methods or HPLC analysis77–79. UV-Visible spectrophotometry is one of the most widespread of anthocyanin spectroscopic methods. The method was also applied for the structural change of anthocyanin under the influence of different physicochemical factors and the process of polymerization of anthocyanins 80. Since their color and stability depend on several factors, much attention has been focused on the development of sensitive and reliable analytical methods for nondestructive quantification of anthocyanins in fruit juices 50.
Fluorescence spectroscopy of anthocyanins has been less studied. In general, anthocyanins are weakly fluorescent in solution, probably due to quenching of the efficient excited state proton transfer to water. Lack of information of anthocyanins luminescence related to their pigments has been limited in the literature80. Fluorescence properties of the chalkone isomer of malvidin 3,5-diglucoside in aqueous solution was studied81. It was found that long-wavelength fluorescence (centered at 495 nm) observed in the fluorescence spectra of cis-chalcone is ascribed to emitting species formed during the excited state of the chalcone form 81,82. The fluorescence of cyanidin and malvidin glycosides in aqueous environment was investigated and found that Cya-3-glc exhibits short-wavelength fluorescence at λmax299 nm which was most effectively excited at 220 nm 80. Similar short-wavelength fluorescence was observed for Cya-3,5-diglc (λmax308 nm) and Mv-3,5-diglc (λmax293 nm) in a binary solvent system. Moreover, the fluorescence approach for measuring anthocyanins and derived pigments in red wine was also reported 77,78.
Determination of Anthocyanins
Effect of pH on Color Change of the Mao Juice
It is widely known that stability of anthocyanins depends on pH of solution. Anthocyanin reversibly changes its color by varying of pH of the solution. In acidic media, anthocyanin exists as the flavylium ion only (AH+). In base solution, the quinonoidal base may be present as an anion A–80. The sample solution was diluted with buffer solutions of pH 1-11. The electrolyte solutions for pH 1-2 were prepared with 0.2MKCl and 0.2 M HCl. Acetate buffer solutions of pH 3-5 were prepared from 0.2 M CH3COOH and 0.2 M CH3COONa. Phosphate buffer solutions of pH 6-8 were consisted of 0.2 M KH2PO4 and 0.2 M K2HPO4 and carbonate buffer solutions of pH 9-11 were prepared from 0.05 MNaHCO3 and 0.1 M NaOH. The certain pH value was measured with a pH meter. Changes in colored sample solutions were measured spectrophotometrically.
UV-Visible absorption spectrum was recorded at wavelength of maximum absorption for each sample. Fluorescence intensity of both Cya-3-glu and PGD-3-glu was measured at 306 nm with the excitation at 277 nm, while the fluorescence intensity of the sample solution was detected at 309 nm with the excitation at 280 nm.
Determination of Anthocyanins by PH–Differential Method
Total anthocyanins were determined using the pH-differential method 71. This method is based on Lambert-Beer’s law: A = εcl. The juice samples were diluted in the solution pH 1.0 (0.025 M KCl) and the acetate (0.4 M) buffer solution pH 4.5. The certain pH values were adjusted to pH 1.0 and 4.5 with a drop wise of strong HCl or NaOH solution. The absorbance of each appropriate dilution of the fruit juiceswas measured at their maximum wavelengths (λmax) in the visible region and at 700 nm for haze background correction. The absorbance (A) of the diluted samples was calculated as
A = (Aλmax – A700)pH 1.0– (Aλmax – A700)pH 4.5
Where, Aλmax is the absorbance at the maximum wavelength in the visible region. The total anthocyanin expressed as Cya-3-gluand PGD-3-gluequivalents were calculated with the following formula:
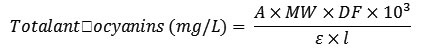
Where, A is the absorbance obtained from pH-differential, including MW 484.84 g/mol for Cya-3-glu and 464.84 g/mol for PGD-3-glu; DF (dilution factor); l(path length) in cm; ε = 26,900 molar extinction coefficient for Cya-3-glu and 31,620 for PGD-3-glu in Lmol–1cm–1; 103 (g-to-mg conversion factor). The total anthocyanins are, however, expressed as mg/100 mL of the juice sample.
Determination of Anthocyanins by UV–Visible Spectrophotometry
Total anthocyanins are calculated as Cya-3-glu equivalent or PGD-3-glu equivalent by means of their calibration curve obtained. Standard solutions of Cya-3-glu (5.0-30 mg/L) in an acidified methanol (1%HCl in methanol) and PGD-3-glu (5.0-25 mg/L) diluted with 1%HCl in deionized water were used for each calibration curve. The absorbance of each sample dilutionwas measured at 528 nm and 497 nm for Cya-3-glu and PGD-3-glu, respectively. Total anthocyanins were expressed mg/100 mL Cya-3-glu and PGD-3-glu equivalent.
Determination of Anthocyanins by Spectrofluorophotometry
Cya-3-glu and PGD-3-glu were chosen as the reference compounds. They were diluted in 12% methanol solution pH 2.0 77–78. This pH was chosen according to preliminary study on the effect of pH on fluorescence intensity of the anthocyanins. Excitation spectra were recorded from 220 nm to 300 nm for the emission at 306 nm, whereas emission spectra were measured between 310 nm and 450 nm with the excitation at 277 nm. For each sample, excitation spectra were also recorded from 220 nm to 300 nm for the emission at 309 nm, whereas emission spectra were measured between 310 nm and 450 nm with the excitation at 280 nm.
Cya-3-glu (1.0-5.0 mg/L) and PGD-3-glu (0.5-2.0 mg/L) were diluted in buffer solution pH 2.0 for plotting of their calibration curves. All samples were diluted with the same solvent. For both standard and sample solutions, the fluorescence spectra were recorded with the excitation/emission of 277/306 nm for Cya-3-glu and the excitation/emission of 282/311 nm for PGD-3-glu by spectrofluorophotometer.
Method Validation for Anthocyanins
The linearity was tested for the concentrationrange of 5-30 mg L–1of anthocyanins standard for UV-Visible spectrophotometry, 0.5-5mg L–1for spectrofluorophotometry, while the dilution factorof the Mao juice samples are 200-1600 folds and 1000-64000 folds forUV-Visible spectrophotometry and spectrofluorophotometry, respectively. The calibrationcurve was constructed and evaluated by its correlationcoefficient. The correlation coefficient (R2) for all thecalibration curves was consistently greater than 0.995 83,84.
Accuracy of the developed method was evaluated by recovery study of total anthocyanins by standard addition method at three spiking levels (low, medium and high of their calibration curve) of each standard of both anthocyanis. The amount of anthocyaninswasestimated by applying the obtained values to the regressionequation of the calibration curve84,85.
Ascorbic Acid
Ascorbic acid (C6H8O6; L-ascorbic acid)) or vitamin Cis an antioxidant, along with vitamin E, β-carotene, and many other plant-based nutrients. It plays an important role in collagen biosynthesis, iron absorption, and immune response activation and is involvedin wound healing and osteogenesis. It also acts as a powerful antioxidant which fights against free-radical induced diseases11. Ascorbic acid is the enolic form of one α-ketolactone. Ascorbic acid solution is easily oxidized to the diketo form referred to as dehydroascorbic acid, which can easily be converted into oxalic acid, diketogulonic acid or threonic acid 86,87.
Antioxidants have aromatic ring structures and are able to delocalize theunpaired electron. Vitamin C (AscH–) in the aqueousphase will directly react withor neutralize hydroxyl, alkoxyl and lipid peroxyl (ROO·) radicalsand form H2O, alcohol and lipid hydroperoxides, respectively. Vitamin C turns to avery stable radical (Asc–•), due to its delocalized structure. Moreover, vitamin C can also neutralize the radical formof other antioxidants such as glutathione radical and vitamin Eradical, and regenerate these antioxidants. Vitamin Citself is readily regenerated from Asc–• with NADH or NADPHdependentreductases88–90.
For the determination of ascorbic acid in foods, the method should apply for both, ascorbic acid and dehydroascorbic acid, to give a total value of vitamin C. Many analytical techniques are mentioning in the literature for the determination of vitamin C in different matrices, such as titrimetric, fluorimetric,spectrophotometric,high-performance liquid chromatographic, or enzymatic methods etc.11.
Spectrophotometric determination of total ascorbic acid is based on coupling with 2,6-dichlorophenol indophenols dye (DCPIP) in different samples of fruits and vegetables. This procedure is one of most simple, accurate and applicable method for determination of total ascorbic acid in fresh foods, such as fruits and vegetables. DCPIP is organic dye with both acid/base and redox properties,which is blue in neutral solution and pink in acidic solution91,92.
Thermal Stability
Thermal stability of antioxidants is veryimportant in food preservation. The effectiveness of antioxidants varies depending on the food and conditionsof processing and storage. The thermal processing of foods involves heating to temperatures ranging from 50oC to 150oC, depending on the pH of theproduct and the desired shelf life93. Some publicationshave been reported as follows.Fischer et al. (2013)94studied on systematic assessment of the factors influencing the anthocyanin stabilityand color retention of pomegranate juices and less complex model solutions with particular focuson the effects of colorless phenolic co-pigments. The stability of putative healthpromotingpolyphenols of pomegranate juices was not markedly affected by thermal treatment. Unexpectedly, the HMF contents only slightly increased upon forced heating. Therefore, the visualappearance does not adequately reflect the quality and storage stability of pomegranate juices.
Jie et al. (2013)95identified thirteen anthocyanins in the purple-fleshed sweet potato cultivar.The enrichmentand degradation kinetics of anthocyanins in these matrices were investigated at 80, 90 and 100oC. Ahigher stability of anthocyanins was obtained in aqueous solutions with pH 3 and 4 and in apple and pearjuices.Volden et al. (2008)96studied the effects of various thermal processing treatments (blanching, boiling and steaming) of red cabbage. Autoro was assessed for the levels of glucosinolates (GLS), total phenols (TP), total monomeric anthocyanins (TMA),L-ascorbic acid (L-AA) and soluble sugars, as well as for the antioxidant potential by the ferric reducing ability power (FRAP) and oxygenradical absorbance capacity (ORAC) assays. There were significant (p<0.05) losses in blanched red cabbage. Boiling gave less extensive reductions: TP,TMA, FRAP, ORAC, L-AA and soluble sugars. Steamingcaused no losses for TP, ORAC, FRAP or soluble sugars. Total GLS were severely affected by processing, with reductions of 64%, 38% and 19% in blanched, boiled andsteamed red cabbage, respectively.
Sadilova et al. (2007)97investigated the structural changes of anthocyanins at pH 3.5 in purified fractions from black carrot, elderberry and strawberry heated over 6 h at 95°C. Degradation products were monitored by HPLC-DAD-MS to elucidate the prevailing degradation pathways. After heating, decline of Trolox equivalent antioxidant capacity (TEAC) was observed in all samples, which was attributed to both anthocyanins and their colorless degradation products following thermal exposure. As deduced from the ratio of TEAC value and anthocyanin content, the loss of anthocyanin bioactivity could not be compensated by the antioxidant capacity of newly formed colorless phenolics upon heating.
Table 1 Antioxidant activity, total phenolics, flavonoids, anthocyanins and ascorbic acid of Mao and other fruit juices
| Juice |
Antioxidant activity |
Total phenolics92 (mg GAE/100 mL) |
Flavonoids (mg CE/L) |
Anthocyanins (mg/L) |
Ascorbic acid92 (mg GAE/100 mL) |
|||||
| Mao |
26.8* |
338 |
19,899 |
578 |
175.3 |
|||||
| Orange98 |
9.2 |
382 |
– |
– |
15.86 |
|||||
| Pomegranate99 |
20.0** |
135 |
– |
– |
17.34 |
|||||
| Red grape100 |
11.1 |
64.3 |
– |
– |
15.18 |
|||||
| Apple100 |
1.20*** |
42.8 |
– |
– |
13.40 |
|||||
| White grape |
– |
33.7 |
– |
– |
14.13 |
|||||
| Apricot101 |
0.974 |
18.6 |
– |
– |
16.20 |
|||||
| Noni92 |
5.85 |
– |
– |
– |
– |
|||||
| Sour cherry |
– |
50.1 |
618.1 |
368.4 |
16.44 |
|||||
| Peach |
– |
28.6 |
– |
– |
15.63 |
|||||
| Sweet cherry74 |
– |
– |
– |
256.6 |
– |
|||||
| Mango |
– |
42.9 |
– |
– |
12.57 |
|||||
| Pineapple |
– |
35.7 |
– |
– |
13.60 |
|||||
| Blackcurrant102 | – | – | 975.1 | 849.8 |
– |
|||||
| Black grape102 |
– |
– |
405.2 |
208.7 |
– |
|||||
| Elderberry103 |
– |
– |
1,776 |
– |
– |
|||||
| Strawberry104 |
– |
– |
– |
13.6 |
– |
|||||
| Raspberry104 |
– |
– |
– |
336.7 |
– |
|||||
-: no data; *BHT by DPPH;**92, 98,99,100TEAC: Trolox equivalent antioxidant capacity; ***100,101AEAC: ascorbic acid equivalent antioxidant capacity;74Jakobek et al., 2007;92Mahdavi et al., 2010;98Gil et al., 2000; 99Burin et al., 2010; 100Pernicea et al., 2009; 101Pisoschi et al., 2009; 102Mitić et al., 2011; 103Garofulić et al., 2012; 104Lee et al., 2005;
Conclusion
Total phenolics including flavonoids and anthocyanins, and ascorbic acid in the fruit juiceproduced from the Mao tree highly related to their antioxidant activity and thermal stability are reviewed, since recent research approaches on the functional foods, in particular the colored fruits and vegetables, have been focused. In analytical aspects, method validation and determination of these mentioned groups of the potential health’s benefit compounds have been increasingly developed. Emphasizing on study of the Mao juice products commercially available in Thai markets, compared with other fruit juices as shown Table 1, are reported.
Acknowledgements
The authors gratefully acknowledge Materials Chemistry Research Center, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, and Sakon Nakhon Rajabhat University, Sakon Nakhon province, Thailand for financial support.
References
- Alonso, A.M., Guillen, D.A., Barroso, C.G., Puertas, B., Garcia, A.J. Agric. Food Chem,2002,50(21), 5832-5836.
CrossRef - Rababah, T.M., Hettiarachch, N.S., Horax, R. J. Agric. Food Chem,2004,52(16), 5183-5186.
CrossRef - Margetts, B., Buttriss, J.In: Plants: Diet and Health Goldberg,G(Ed). Blackwell Publishing: Oxford, UK,2003, 49-64.
- Samappito, S., Butkhup, L. Pak J Biol Sci,2008, 11, 974-981.
CrossRef - Gharras, H. E.l.Int. J. Food Sci Tech,2009,44(12), 2512-2518.
CrossRef - Gardner, P.T., White, T. A. C., McPhail, D. B., Duthie, G.G. Food Chem, 2000, 68, 471-474.
CrossRef - Aklima, J., Mojumder, S., Sikdar, D.Int Food Res J,2014,21(1), 119-124.
- Buthup, L., Samappito, S. J. Fruit Ornam. Plant Res, 2011,19(1), 85-99.
- Butkhup, L., Samappito, S. Pak J BiolSci,2008,11(7), 996-1002.
CrossRef - Puangpronpitag, D., Areejitranusorn, P., Boonsiri, P., Suttajit, M., Yongvanit, P. J Food Sci,2008,73(9), 648-653.
CrossRef - Pisoschi, A.M., Danet, A.F., Kalinowsk, S. J Autom MethodsManag Chem, 2008, 8.
- Gulcin, I., Bursal, E., Sehitoglu, M.H., Bilsel, M., Goren, A.C. Food Chem Toxicol,2010,48, 2227-2238.
CrossRef - Hassan, S.M., Al Aqil, A.A., Attimarad,M. Adv Med Plant Res,2013,1, 8-24.
- Kalita, P., Tapan, B.K., Pal, T.K., Kalita, R. J. Drug Deliv. Ther, 2013, 3(4), 33–37.
- Kukongviriyapan, U., Kukongviriyapan, V., Pannangpetch, P., Donpunha, W., Sripui, J., Sae-Eaw, A., Boonla, O. Nutr, 2015, 7, 6179-6194.
- Association of Official Analytical Chemists.AOAC,1995, 16(2).
- Nuengchamnong, N., Ingkaninan, K. Food Chem,2010,118, 147–152.
CrossRef - Hansakul, P., Dechayont, B., Phuaklee, P., Prajuabjinda, O., Juckmeta, T., Itharat, A. Trop J Pharm Res,2015,14(4), 627-634.
CrossRef - Beynon, R. J., Easterby,J.S. IRL Press at OxfordUniversity Press,NY. 1996.
- Diplock, A.T, Charleux, J.L., Crozier-Willi, G, Kok, F.J., Rice-Evans, C., Roberfroid,M., Stahl, W., Viña-Ribes, J. Br J Nutr,1998,80 (Suppl 1), S77-S112.
CrossRef - Valko, M., Leibfritz, D., Moncol, J, Cronin, M.T., Mazur, M., Telser, J. Int J Biochem Cell B, 2007,39(1), 44-84.
CrossRef - Lobo, V., Pati, A., Phatak, A., Chandra, N. Rev, 2010,4(8),118–126.
- Young, I.S, Woodside, J.V. J Clin Pathol, 2001,54, 176–186.
CrossRef - Khalaf, N. A., Naik, R.R.,.Shakya, A.K., Shalan,N., Al-Othman, A. Orient.J. Chem,2015, 31(4), 1923-1928.
CrossRef - Apak, R., Gorinstein, S., Böhm, V., Schaich, K.M., Özyürek, M., Güçlü, K. Pure Appl. Chem,2013,85(5), 957–998.
CrossRef - Antolovich, M., Prenzler, P.D., Patsalides, E., McDonald, S., Robards, K. Analyst,2002, 127, 183–198.
CrossRef - Jadhav, S.J., Nimbalkar, S.S., Kulkarni ,A.D., Madhavi, D.L. In Food Antioxidants: Technological,Toxicological and Health Perspectives, New York,1996, 5–64.
- Leclercq, C., Arcella, D., Turrini, A. Food Chem. Toxicol, 2000,38(12), 1075-1084.
CrossRef - Maziero, G.C., Baunwart, C., Cecilia, M., Toledo, F. Braz. Food Addit. Contam, 2001, 18, 365-373.
CrossRef - Shahidi, F., Janitha, P.K., Wanasundara, P.D. FoodSci.Nutr,1992, 32(1), 67-103.
- Huang, D., Ou, B., Prior, R.L. J. Agric. Food Chem,2000, 53, 1841-56.
CrossRef - Prior R L, Wu X, Schaich K.J. Agric.Food Chem, 2005, 53, 4290-4302.
CrossRef - Blois MS. Nature, 1958, 181, 1199–1200.
CrossRef - Cheeseman KH, Slater TF. Br Med Bull,1993, 49, 481–93.
CrossRef - Shi, H., Noguchi, N., Niki, E. WoodheadPublishing Limited, Cambridge,2001, 147-158.
- Flora, S.J.S. Oxid Med Cell Longev, 2009,2(4), 191–206.
CrossRef - Chang, H-Y., Ho, Y-L, Sheu, M.J., Lin, Y-H., Tseng, M-C., Wu, S-H., Huang, G-J., Chang, Y-S.Bot Stud, 2007, 48, 407-417.
- Gupta, J., Gupta, A. Orient. J. Chem.,2015,31(Spl Edn.), 231-235.
- Liang, N, Kitts, D.D. Molecules,2014, 19(11), 19180- 19208.
CrossRef - Busuricu, F., Negranu-Pârjol, T., Balaban, D.P., Popescu, A., Anghel, A. Innov Rom Food Biotechnol, 2008, 2(2),10-18.
- Mehdi, M.M., Rizvi, S.I.Anal. Biochem,2013,436, 165–167.
CrossRef - Bhanja, T., Kumari, A., Banerjee, R. Bioresour. Technol, 2009, 100, 2861–2866.
CrossRef - Dehghan, G., Shafiee, A., Ghahremani, M., Ardestani, S., Abdollahi, M. Pharm Biol,2007,45(9), 1–9.
CrossRef - Floegel, A., Kim, D.O., Chung, S.J., Koo, S.I., Chun, O.K. J Food Compost Anal,2011, 24, 1043–1048.
CrossRef - Ozgen, M., Reese, R.N, Artemio, Z., Tulio, J.R., Scheerens, J.C., Raymon Mille, A. J. Agric. Food Chem, 2006, 54, 1151-1157.
CrossRef - Sharma, P., Singh, R.P. J. Food Technol,2013,8(2), 83-101.
CrossRef - Benzie, I.E.F., Strain, J.J.Anal Biochem,1996,239, 70-76.
CrossRef - Ayub Ali1, M., InaotombiDevi, L., Nayan, V., Victoria Chanu, K., Ralte, L. Int J Biol Pharm Res,2010, 1(2), 76-81.
- Abbasi, M.A., Saleem, H., Rehman, A-ur., Riaz, T., Ajaib, M. BJPR,2013,3(2), 202-216.
CrossRef - Shalaby, E.A., Shanab, S.M.M. Afr J Pharm Pharmacol,2013, 7(10), 528-539.
CrossRef - Pulido, R., Bravo, L., Saura-Calixto, F. J AgricFood Chem,2000, 48, 3396-3402.
CrossRef - Cao, G., Prior,R.L.In Handbook of Antioxidants :New York, 2001, 47-55.
- Erel, O. Clin Biochem,2004,37, 277-285.
CrossRef - Hu, M.Mol Pharm, 2007,4, 803.
CrossRef - Ossipova ,S., Ossipov, V., Haukioja, E., Loponen ,J., Pihlaja, K. Phytochem Anal,2001, 12, 128–133.
- Pelozo, M.I.G., Cardoso, M.L.C., Mello, J.C.P. Biol. Technol,2008,51, 447–451.
- Karamian, R., Ghasemlou, F.Intl J Agri Crop Sci,2013,5(3), 305-312.
- Archivio, M.D., Filesi, C., Benedetto, R.D., Gargiulo, R., Giovannini, C., Masella, R. Ann Ist Super Sanità, 2007, 43(4), 348-361.
- Abu Bakar, M.F., Mohamed, M., Rahmat, A., Fry, J. Food Chem,2009,113, 479-483.
CrossRef - Tarozzi ,A., Morroni Hrelia, S., Angeloni, C., Marchesi, A., Cantelli-Forti, G.Hrelia, P. Neurosci Lett, 2007, 424: 36-40.
CrossRef - Azevedo, J., Fernandes, I., Faria, A., Oliveria, J., Fernandes, A., De Freitas, V. Mateus, N. Food Chem,2010, 119, 518-523.
CrossRef - Bishayee ,A., Mbimba, T., Thoppil, R.J., Háznagy-Radnai, E., Sipos,P., Darvesh, A.S. J Nutr Biochem,2011,22, 1035-1046.
CrossRef - Rakić, V.P., Ota, A. M., Može Bornšek, Š.F., Miljković, M.N, Sokolović, D.T, Poklar Ulrih, N.E. Adv Technol, 2014, 3(2), 5-9.
- Sarić, A., Sobocanec, S., Balog, T., Kusić, B., Sverko, V., Dragović-Uzelac, V., Levaj, B., Cosić, Z., Macak Safranko, Z., Marotti ,T. Plant Foods Hum Nutr, 2009,64, 231-237.
CrossRef - Sun, C., Zheng, Y., Chen, Q., Tang, X., Jiang, M., Zhang, J., Xian, L. FoodChem,2012, 131, 1287-1294.
CrossRef - Tremblay, F., Waterhouse, J., Nason, J., Kalt, W.J Nutr Biochem,2013, 24, 647-655.
CrossRef - Yang, X., Yang, L., Zheng, H. Food Chem Toxicol,2010,48, 2374-2379.
CrossRef - Yao, N., Lan, F., He, R-R, Kurihara, H. J Agric. Food Chem,2010, 58, 4731-4736.
CrossRef - Arnok, P., Ruangviriyachai, C., Mahachai, R., Techawongstien, S., Chanthai, S. Int Food Res J,2012,19(1), 235-243.
- Bondre, S., Patil, P., Kulkarni, A., Pillai, M.M. Intl. J. Adv. Biotec. And Res,2012, 3(3), 698-702.
- Lee. J., Rennaker, C., Wrolstad, R.E. Food Chem,2008,110, 782-786.
- Tang, K., Li, Y., Han, Y., Han, F., Li, J., Nie, Y., Xu, Y. J. Sci. Food Agr, 2014, 94, 2472-2481.
CrossRef - Cevallos-Casals, B.A, Cisneros-Zevallos, L. Food Chem,2004,86, 69-77.
CrossRef - Jakobek, L., Šeruga, M., Medvidović-Kosanović, M., Novak, I. Deutsche Lebensmittel-Rundschau,2007,103, 58–64.
- Roy, H.J., Lundy, S., Eriksen, C., Kalicki, B. Anthocyanins. Pennington Biomedical Research Center, 2009, 4
- Lopes, T.J, Yaginuma, S.R., Quadri, M.G.N., Quadri, M.B. Braz. Arch. Biol. Technol, 2011, 54(6), 1349-1356.
CrossRef - Agati, G., Matteini, P., Oliveira, J., Freitas, V.D, Mateus, N. J. Agric. Food Chem, 2013,61, 10156-10162.
CrossRef - Agati, G., D´Onofrio, C., Ducci, E., Cuzzola, A., Remorini, D., Tuccio, L. J. Agric. Food Chem,2013, 61,12211- 12218.
CrossRef - Rakić, V.P, Ota, A.M, Skrt ,M.A, Miljković, M.N, Kostić, D.A, Sokolović, D.T, Poklar Ulrih, N.E. Hem. Ind,2015, 69(2), 155-163.
CrossRef - Drabent, R., Pliszka, B., Olszewska, T. J. Photochem. Photobiol.B, Biol,1999, 50, 53-58.CrossRef
- Figueiredo, P., Pina ,F., Vilas-Boas, L., Macanita, A.L. J. Photochem. Photobiol.A. Chem, 1990, 52(3), 411-424.
CrossRef - Lima, J.C., Danes, P., Figueiredo, P., Pina, F.S, Macanita, A. J. Photochem. Photobiol.A. Chem,1994,59, 412-418.
CrossRef - ICH, Q2 (R1).International Conference on Harmonization, 2005.
- Patel Satish, A., Patel Natvarlal, J. JAPHAC, 2011, 01(07), 127-131.
- Lakshmi, K.S., Rajesh, T.Iran. Chem. Soc, 2011, 8(1), 31-37.
CrossRef - Dehmolaei, A., Vadi, M. Orient. J. Chem,2014, 30(1),233-236.
CrossRef - Dua, J., Cullena, J.J., Buettner, G.R. Biochim. Biophys.Acta – Reviews on Cancer, 2012, 1826(2), 443-457.
- Defeudis, F.V., Papadopoulos. V., Drieu, K.Fundam Clin Pharmacol, 2003,17, 405–17.
CrossRef - Hossain, M.A., Asad, K. J Biol Chem,1985, 260, 12920–6.
- Lü, J.M., Lin, P.H., Yao, Q., Chen, C. J Cell Mol Med,2010, 14(4), 840-860.
CrossRef - Fadhel, D.H. JNUS, 2012,15(3), 88-94.
- Mahdavi, R., Nikniaz, Z., Rafraf, M., Jouyban, A. Pak. J. Nutr, 2010, 9(10), 968- 972.
CrossRef - Patras, A., Brunton, N.P., O’Donnell, C., Tiwari, B.K. Trends Food Sci Tech, 2010,21, 3–11.
CrossRef - Fischer Ulrike, A., Carle, R., Kammerer Dietmar, R. Food Chem,2013,138, 1800–1809.
CrossRef - Jie, L., Xiao-ding L., Yun, Z., Zheng-dong, Z., Zhi-ya, Q., Meng, L.,Zhu, S-H., Liu, S.,Wang, M., Qu, L. Food Chem, 2013, 136, 1429–1434.
CrossRef - Volden, J., Borge, G.I.A., Bengtsson, G.B., Hansen, M., Thygesen, I.E., Wicklund, T. Food Chem,2008,109, 595–605.
CrossRef - Sadilova, E., Carle, R., Stintzing, F.C. Mol. Nutr. Food Res, 2007, 51, 1461 – 1471.
CrossRef - Gil, M. I., Tomás-Barberán, F. A., Hess-Pierce, B., Holcroft, D. M., Kader, A.A. J Agric Food Chem, 2000, 48(10), 4581-4589.
CrossRef - Burin, V. M., Falcão L. D., Gonzaga, L. V., Fett, R., Rosier, J. P., Bordignon- Luiz, T. Ciênc. Tecnol.Aliment Campinas, 2010, 30(4), 1027-1032.
CrossRef - Pernicea Pernicea, R., Borrielloa, G., Ferracanea, R., Borrelli, R. C., Cennamo, F.,Ritieni, A. Food Chem,2009, 112, 545–550.
CrossRef - Pisoschi, A. M., Cheregi , M. C., Danet, A. F. Molecules,2009,14, 480-493.
CrossRef - Mitić, M.N., Obradović, M.V., Kostić, D. A., Nasković, D.Č., Micić,R.J. Hem. Ind. 2011, 65(5), 611–619.
CrossRef - Garofulić, L.E., Kovačević Ganić, K., Galić, L., Dragović-Uzelac, V., Savić, Z. CroatJ Food Technol Biotechnol Nutr,2012, 7 (Special Issue), 9-13.
- Lee, J., Durst, W.R., Wrolstad, R.E.J. AOAC Int, 2005, 88(5), 1269-1278.

This work is licensed under a Creative Commons Attribution 4.0 International License.









