Green Chemistry Formation of Stable Ag Nanoparticles (Agnps) in Isopropanol Solvent
SyukriArief1, Putri Hidayani1, Lusi Aferta1, Zulhadjri1, Takayuki Ban2 and Yutaka Ohya2
1Department of Chemistry, Faculty of Mathematic and Sciences – Andalas University, Kampus Limau Manis, Padang – Indonesia.
2Department of Chemistry and Biomelecular Science, Faculty of Engineering, Gifu University, Yanagido 1 – 1, Gifu – Japan.
Corresponding Author E-mail: syukriarief@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330109
Article Received on :
Article Accepted on :
Article Published : 16 Feb 2017
Several bioreduction processes become appreciation for the synthesis of silver nanoparticles (AgNPs) because of their multiple applications, cost effective and eco-friendly. The main problems in the synthesis of AgNPs are colloidal systems agglomeration and damage due to precipitation. In this study,it has been investigated the use ofUncariaGambirRoxbleaf extract as bioreductorand capping agent on the synthesis of AgNPsand isopropanol as a solvent. XRD studies showed that the AgNPsare crystalline fcc (face center cubic) without another phase and AgNPs size in isopropanol solution was smaller than aqueous solution. TEM images indicated that the obtained AgNPs are predominantly spherical shape, with sizes in the range of 10 to 30nm.
KEYWORDS:Silver nanoparticles; bioreductor; isopropanol solvent; Uncaria Gambir Roxb
Download this article as:| Copy the following to cite this article: Arief S, Hidayani P, Aferta L, Zulhadjri Z, Ban T, Ohya Y. Green Chemistry Formation of Stable Ag Nanoparticles (Agnps) in Isopropanol Solvent. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Arief S, Hidayani P, Aferta L, Zulhadjri Z, Ban T, Ohya Y. Green Chemistry Formation of Stable Ag Nanoparticles (Agnps) in Isopropanol Solvent. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=30221 |
Introduction
In recent years, research on the synthesis of metal nanoparticles has been widely discussed and has become a major topic due to their new or improved chemical, optical, electric, and magnetic properties compared to their bulks [1]. Among them, silver nanoparticles (AgNPs) have been a focus of many researchers not only because of their properties but also because silver nanoparticles have been widely used as an antimicrobial property [2].Their properties strongly depend on their size and shape, and therefore it is very important to be able to finely control the morphology of the nanoparticles.
Several methods have been used in the formation of AgNPs such as the reduction in solution, electroless, photochemical reduction, hydrothermal, and assisted-microwave synthesis. So far, several weaknesses have been found, including the need for sophisticated and more expensive equipment.They also have high toxicity properties and more particularly difficult to obtain a stable colloid.
Agreen synthesis method is an attractive option at this time where the synthesis of AgNPs uses various extracts of the leaves of plants including the most leaves that have flavonoid compounds in the presence of polyphenols structure such as Rumexhymenasepalus [3], Artocarpusheterophyllus [4], Camellia Sinensis [5], Mollugonudicaulis[6] and Azadirachtaindica [7] which can reduce Ag(I) to Ag(0). Ramachandran et al [8] and SiavashIravani [9] have reviewed various kinds of plant extracts that have been used in the synthesis of metal nanoparticles, especially Ag nanoparticles. In general, the resulting nanoparticles of this process have size in the range of nanosize with spherical and triangular structures [10-12].
In the case of metal oxides, solvent is a factor that determines the stability and also controls the size and shape of the particles [13]. In our previous studies, it has been reported that the use of leaf extracts bioreducinggambierwas able to act as reducing agent in the formation of silver nanoparticles in aqueous solvent. AgNPs with sizes of 25 – 40 nm have been obtained with the help of a hydrothermal process [14]. In this study we report biosynthesis of AgNPs by reduction of silver nitrate (AgNO3) in isopropanol solution using isopropanol – Gambier leaf extract in order to control the shape and size of silver nanoparticles.
Materials And Methods
Plant material and preparation of extracts
All chemicals used for this reaction were of analytical grade and obtained from Merckwithout any further purifications. Gambir leaves were collected from agricultural field located at Payakumbuh – West Sumatera, Indonesia. Fresh leafs of Gambir were washed thoroughly with double distilled water. Dried leaf were incised and crushed into small pieces. Approximately 5 g of those finely Gambir were weighted and transferred into a 200 ml beaker containing 50 ml isopropanol, mixed well and boiled for 30 minutes. The extract obtained was filtered through filter paper and the filtrate was diluted and collected to 250 ml with isopropanol. The solution was kept in refrigerator for further use.
Synthesis of AgNPs
The Ag Nanoparticles from Gambier leaves extract was prepared as previously described method with slightly modifications [14]. Isopropanol solution (5 mM) of silver nitrate (AgNO3) was prepared and used for the synthesis of silver nanoparticles. An amount of 10 ml of extract was added to 40 ml of 5 mM AgNO3 solution. After addition of extracts, the color of the AgNO3 solution altered to brown. The effect of reaction times on the synthesis of silver nanoparticles was carried out and evaluated every 10 minutes.The precipitate was separated by high speed centrifugation at 6000 rpm. The separated solid mass was washed three times with ethanol to remove the organic alcohol soluble impurities. Product was dried in oven at 50C for 2–3 h. Finally, it was transferred into light resistant containers for further characterization.
Characterization
UV–vis spectroscopic studies were carried out on a Thermo spectrophotometer. The sample for XRD measurement was prepared by depositing a dried sample on a slide and the diffraction pattern was recorded on a PANalytic X’PERT-PRO X-ray spectrometer. The size of the nanoparticles was calculated by the Scherer’s equation. High resolution-transmission electron microscopic (HR-TEM) measurements were done using a Shimadzu CTLA-4 microscope.
Results And Discussions
UV–vis analysis
The addition of the extract into 5 mM of AgNO3 solution changed color from pale yellow to brownish within 5 minutes. The color is more intense with increasing time of reaction. The change in color is due to the strong absorption of visible light due to excitation of the nanoparticle surface plasmons [15]. This is a strong indication of the formation of silver nanoparticles. In Figure 1, the curves demonstrate the absorption of the the plant extract (A), pure AgNO3 solution (B), and a sample of AgNPs with reaction time for 4 h (C). The polyphenol peak (observed in the Gambir extract) is also clearly visible around 278 nm, while the UV-Vis spectra of the AgNO3 solution has a peak around 305 nm, as expected for Ag+ ions. These spectral studies identify the formation of stable nanoparticles of silver (insert: higest peak for one week). The maximum absorption shows the appearance of a single and strong surface plasmon resonance band absorption peak centered at about 427 nm, which indicates that these particles are isotropic in shape and relatively uniform in size.
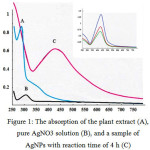 |
Figure 1: The absorption of the plant extract (A), pure AgNO3 solution (B), and a sample of AgNPswith reaction time of 4 h (C) |
X-Ray diffraction (XRD)
The XRD analysis is performed to confirm the crystalline nature of AgNPs that prepared by using Gambier extract as bioreductor. Various Bragg’s reflections are clearly visible in silver XRD pattern, which are corresponding to the (111), (200), (220) and (311) set of lattice planes, and also agreed by joint committee of powder diffraction standard (JCPDS) Card No- 04-0783 (Fig. 2).On the other hand, the difference in solvent gives different peak sharpness and heights. Peaks obtained fromaqueous solution aresharper and higher than isopropanol solution. Based on the equation Scherer, a sharp and heightpeak giveslarger crystal size than the width and low peak. This will be proved later by TEM.
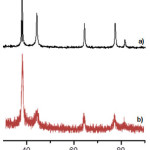 |
Figure 2: XRD patterns of synthesized AgNPsusing different solvent, a) in aqueous solution, b) Isopropanol solution. |
Transmission Electron Microscope (TEM)
The typical TEM images of AgNP-Gambier Isopropanol and aqueous solution are given in Fig. 3.The images show that the nanoparticles synthesized using Isopropanol as solvent are almost spherical and slightly polydispersed. The size of AgNPs is ca. 10–25 nm according to the TEM image, and that is consistent with the published data for the synthesis of AgNPs by the chemical reduction method [16]. The TEM images show good stabilization of silver nanoparticles due to interaction with the Gambier – isopropanol solution. Based on ionic strength, water media is more powerful than isopropanol media. Their electrophoretic mobility is freer in aqueous media due to faster growth of nanoparticles. Organic media (isopropanol solvent) is absorbed on to these nanoparticles significantly reducing the aggregation and improving its AgNPs stability.
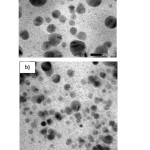 |
Figure 3: TEM images of the AgNPs at different solvents, a) in aqueous solution, b) Isopropanol solution |
Conclusion
We have demonstrated a green chemistry approach for the synthesisof silver nanoparticles at ambient reaction conditions usingUncaria GambierRoxbleaf extract and isopropanol solvent. The leaf extract acts as reducingagent as well as stabilizing/capping agent. These biosynthesized AgNPs are highly stable for several weeks in isopropanol solution. XRD studies show that the AgNPsare crystalline fcc (face center cubic) and particles size is of 20 nmusing isopropanol solution. TEM images indicate that the obtained AgNPs are predominantly spherical shape, with sizes in the range of 10 to 30nm.
Acknowledgements
The authors would like to thank the Higher Education Ministry of Indonesia via Andalas University that has funded this research through Grants of Cooperation Foreign Affairs and International Publications with contract No. 020/SP2H/LT/DRPM/Il/2016.
References
- Schmid, G.(ed.), Nanoparticles : From Theory to Application, 2nd Ed., Wiley-VCH, Singapore,2010.
- David, P.Silver Nanoparticles, Published by In-The, India, 2010.
- Leon, E. R.; Palomares, R. I.; Navarro, R. E.; Urbina, R. H.; Tanori, J.; Palomares, C. I.; Maldonado, A.Nanoscale Res.Let.2013, 8, 318 -324.
CrossRef - Thirumurugan, A.; Tomy, N.A.; Ganesh, R.J.; Gobikrishnan, S. Der Pharma Chemica, 2010, 2(6), 279-284
- Loo, Y.Y.; Chieng, B.W.; Nishibuchi, M.; Radu, S. Int. J. Nanomedicine, 2012, 7, 4263-4267
- Anarkali, J.R.; Vijayanti, D.; Rajathi, K.; Sridhar, S. Indo American J. of Pharm. Res, 2012, 4, 1436 – 1442.
- Shankar, S.S.; Rai, A.; Ahmad, A.; dan Sastry, M. J. Colloid Interface Sci., 2004, 275(4), 496-502.
CrossRef - Ramachandran R.; Krishnaraj C.; Stacey L. H.; Soon-Il Y.; Thangavel K.Ind. Crops Prod.,2015, 70, 356–373
CrossRef - Siavash I., Green Chem., 2011, 13, 2638-2650
CrossRef - Singh C.; Baboota, R. K.; Naik, P. K.; Singh H.Adv. Mater. Lett.2012, 3(4): 279-285.
CrossRef - Leela A. and Vivekanandan M.African J.of Biotech.,2008, 7(17): 3162-3165.
- Zargar M.; Hamid, A. A.; Bakar, F. A.; Shamsudin, M. N.; Shameli K.; Jahanshiri F.; Farahani F.Molecules , 2011, 16: 6667-6676.
CrossRef - Kraynov A. and Thomas E.M. Application of Ionic liquid in Science and Technology, Edited by Scott T. H., In-tech, Croatia, 2011
- SyukriA.; Vivi G.; Diana V.W.; Zulhadjri Z.;Ban T.andOhy Y. J. Chem. Pharm. Res.2015, 7(9S), 189-192
- Ray PC,Chem Rev.,2010, 110, 5332–5365
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









