Effect of Eggshell on Mechanical Properties of Epichlorohydrin Cross-linked Chitosan/Eggshell Composites
Rahmi, Marlina and Nisfayati
Chemistry Department, Syiah Kuala University, Banda Aceh 23111, Indonesia.
Corresponding Author E-mail: rahmi@fmipa.unsyiah.ac.id
DOI : http://dx.doi.org/10.13005/ojc/330156
The effect of eggshell particles on the mechanical properties of epichlorohydrin crosslinked chitosan/eggshell composites had been studied. The content of eggshell particles in each composites was varied from 2.5 to 13.4% (w/w). The tensile strength was 60% improved, compared to the control chitosan showed the optimum tensile strength. The improvement tensile strength was 60% comparing with control chitosan. XRD patterns exhibited the decrease in peak intensity of chitosan at 2q = 20o due to te addition of epichlorohydrin as a crosslinking agent of chitosan and incorporation of eggshell particles to crosslinked chitosan. The peak intensity at 2q = 44owas increased due to the addition of eggshell particles. Scanning elecron microscopy images confirmed the eggshell particles was dispersed into chitosan matrix.
KEYWORDS:eggshell; chitosan; epichlorohydrin; composite
Download this article as:| Copy the following to cite this article: Rahmi R, Marlina M, Nisfayati N. Effect of Eggshell on Mechanical Properties of Epichlorohydrin Cross-linked Chitosan/Eggshell Composites. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Rahmi R, Marlina M, Nisfayati N. Effect of Eggshell on Mechanical Properties of Epichlorohydrin Cross-linked Chitosan/Eggshell Composites. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=29749 |
Introduction
Biopolymers have received much attention in many applications due to their environmental friendly properties However, the development of biopolymers with performance equals to common synthetics polymers is still in open challenge. chitosan-based materials seem to be an attractive source for development of biodegradable polymers. Chitosan can be considered as a good replacement for synthetic polymers due to its biodegradability, availability, non-toxicity, and low cost materials.
Chitosan is a partially deacetylated derivative of chitin. Chitin can be found in fungal species, insects and crustaceous shells. The most abundant natural polysaccharide after cellulose is chitosan4. Chitosan is a b (1®4) linked linear biopolymer that contains 80% poly(D-glucosamine) and 20% poly(N-acetyl-D-glucosamine)5. The high content of amino and hydroxyl groups of chitosan favorsthis biopolymer modification. Native chitosan itself has poor mechanical properties and soluble in most dilute organic acids, such as formic and acetic acids. In order to improve its properties, various methods had been developed to enhance the chitosan positive characteristics, such as by using crosslinking agents or adding fillers. The treatment using crosslinking agents creates highly resistant dissolution resistance of polymer due to the formation of new linkages between the chitosan chains. The crosslinking procedures can be conducted by reacting chitosan with different crosslinking agents such as polyethylene glycol diglycidyl, ethylene glycol diglycidyl ether, glutaraldehyde and epichlorohydrin6-10.
Another method to improve the polymer properties is filler addition. Hassan, S. B. and V.S. Aigbodion (2015) had used eggshell as a filler in the reinforcement of metal-matrix composites11. Eggshell is one of by-products from food industries and restaurants which is daily produced in a huge amount. The huge amount of eggshell will cause environmental pollution. The utilization of eggshell for composite preparation will increase the added value of eggshell and reduce the environmental pollution. Eggshell contains 94% of calcium carbonate, 1% of calcium phosphate, 1% of magnesium carbonate and 4% of organic matter. The use of eggshell as a filler to improve mechanical properties of polymers has some advantages, such as readily available, low cost, and naturally renewable. The chemical composition and availability of eggshell make it become a potential source of filler for the composite polymers.
Therefore, the purpose of this study is the improvement of mechanical properties of chitosan polymer by the addition of eggshell particles to the chitosan that had been crosslinked by epichlorohydrin, to result in a biodegradable, renewable and low cost material for the replacement of synthetic polymers. In this study,composites were prepared by various eggshell contents. The characterization of composites was done by using tensile test, FTIR, SEM and XRD.
Materials and Methods
Eggshell was collected from some restaurants in Banda Aceh, Indonesia. Chitosan (deacetylation value: 75.0 to 85.0%) and epichlorohydrin were supplied by Tokyo Chemical Industry Co., Ltd. Japan and used as received.
Preparation of eggshell particles
Collected eggshell was cleaned by washing it with hot distilled water. The membranes of eggshell were removed. The cleaned eggshell was dried at 50°C for 5 h and grounded into powder using ball milling machine at 250 rpm
Preparation of Epichlorohydrin Crosslinked Chitosan/Eggshell Composites
The epichlorohydrin crosslinked chitosan/eggshell composite was prepared by phase inversion method. Chitosan (1 g) was dissolved by adding it to a 250 mL beaker containing 100 mL of acetic acid solution (2%). In order to complete dissolvation, the mixture was magnetically stirred at 500 rpm for 3 h. An aliquot of 0.8 mL epichlorohydrin was added to the chitosan solution and magnetically stirred at 500 rpm for 1 h. Then eggshell particles were added to the viscous solution and homogenized for 3 h. Chitosan film was then cast by applying the epichlorohydrin crosslinked chitosan/eggshell suspension onto petri dish and dried for 24 h at room temperature. Dried composite film was peeled off from petri dish and then stored.
Results and Discussion
Mechanical properties of the epichlorohydrin crosslinked chitosan/eggshell composites
The tensile strength of epichlorohydrin crosslinked chitosan/eggshell composite was observed as a function of the eggshell addition ranging between 2.5 to 13.4 % (w/w) (Fig. 1). The tensile strength of pure chitosan was found to be 12.30 kgf/mm2 and the composites with addition 2.5; 9.3 and 13.4% (w/w) eggshell particles increased the tensile strength up to 13.90; 16.80 and 19.70 kgf/mm2 respectively. The addition 13.4% (w/w) of eggshell improved the tensile strength 60% compared to pure chitosan.
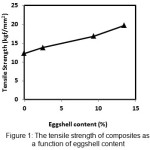 |
Figure 1: The tensile strength of composites as a function of eggshell content |
The tensile strength values of the epichlorohydrincrosslinkedchitosan/eggshell composites increased due to the favorable filler-matrix interactions. The filler-matrix interaction was the interaction betweenthe anionic carbonate groups of eggshell (filler) and the cationic amine groups of chitosan (matrix). This interactionmight favor a good interface between the matrix and filler and lead to high tensile strenght values of the composites. Effective stress transfer at the interface of the filler-matrix also induced the reinforcing of composites.
Fourier Transform Infrared Spectroscopy
Fig. 4 demonstrates the FTIR spectra of chitosan, eggshell and crosslinked chitosan/eggshell composite. As seen in Fig. 2(a) the characteristic absorption bands of the streching of –OH and –CH2OH vibrations of chitosan appeared at 3250-3500 cm-1, overlapped with streching –NH2 (3400- 3500 cm-1) and –NH secondary amides vibrations (3280-3300 cm-1). The absorption bands at 2925 cm-1 and 2855 cm-1 showed the C-H streching vibration of the polymer backbone. The absorption bands at 1084 cm-1 was related to the stretch vibrations of C-O. The absorption bands appeared at 1633 cm-1 and 1589 cm-1related to amide I and amide II vibrational mode, respectively. Primary alcoholic group in chitosan was assigned at absorption band of 1399 cm-1.
 |
Figure 2: FTIR spectra of (a) chitosan (b) eggshell and (c) epichlorohydrin crosslinked chitosan-eggshell composite. |
Fig. 2(b) showed the typical absorption bands of calcite (CaCO3) as main component of eggshell at 1405, 871 and 713 cm-1. Asymmetric streching vibration of carbonate moiety appeared at absorption band of 1405 cm-1. The absorption band at 871 cm-1 was related to in-planebending vibrations of carbonate moietyand the absorption band at 713 cm-1was related to out-of-plane bending vibrations of carbonate moiety. These spectra are in agreement with those obtained by different authors12-13.The occurence of corresponding absorption bands for eggshell (1405, 871 and 713 cm-1), epichlorohydrin and chitosan in the FTIR spectrum of epichlorohydrin crosslinked chitosan/eggshell composite (Fig. 2c.) confirmed their combination within composite.
Scanning Electron Microscopy (SEM)
SEM was used for the characterization of materials from the initial one to the composite. Scanning electron micrographs of chitosan chitosan, eggshell particles and epichlorohydrin crosslinked chitosan/eggshell were shown in Fig. 3. Scanning electron micrographs of chitosan (Fig. 3(a) and (b)) appeared as non-particulate and smooth structure. After crosslinking and addition of eggshell particles(Fig. 3(e) and (f)), the surface exhibits a large number of flower-likeparticles. Scanning electron micrographs of eggshell particles (Fig. 3(c) and (d)) exhibited particle size range of 5-10mm. Scanning electron micrographs of epichlorohydrin crosslinked chitosan/eggshell showed eggshell particles distributed on the surface of chitosan matrix.
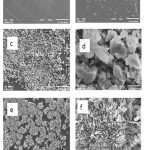 |
Figure 3: Scanning electron micrographs of chitosan (a,b), eggshell particles (c,d), and epichlorohydrin crosslinked chitosan/eggshell composite (e,f) |
X-Ray Diffraction (XRD)
XRD characterization was used for structural analysis of eggshell, chitosan and epichlorohydrin crosslinked chitosan/eggshell composite. Fig. 4 showed the diffractograms of eggshell, chitosan, and epichlorohydrin crosslinked chitosan/eggshell composite.
Diffractogram of eggshell exhibited characteristic peak of calcite (CaCO3) at 2q=28° (Fig. 4(a)).The diffractogram of chitosan exhibited crystalline peak at 2q=20°as shown in Fig. 4(b). Paulino, et. al. (2008) also reported that the characteristic peak for chitosan was in range of 2-Theta=20-23. Due to the formation of crosslinked chitosan and incorporation of eggshell to crosslinked chitosan, a decrease in peak intensity at 2q=20 was observed in diffractogram of epichlorohydrin crosslinked chitosan/eggshell composite (Fig. 4(c)). The degree of crystallinity of composite was considerably lower than that native chitosan because chitosan crystals were destroyed in the processing.
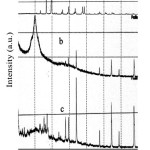 |
Figure 4: XRD patterns of (a) eggshell (b) chitosan and (c) epichlorohydrin crosslinked chitosan-eggshell composite. |
The peak of calcite after treatment with chitosan and epichlorohydrin at 29° disappeared and high intensity peak appeared at 44°, indicating that phase transition of CaCO3 from calcite to vaterite structure (Fig. 4(c)). The chemical composition of vaterite is the same as calcite. But in terms of symmetry, they have different crystal structure, orientation of CO3 ions, and coordination environment of Ca ions15.
Conclusions
It was observed that eggshell particles succesfully incorporated in epichlorohydrin crosslinked chitosan. The microstructure analysis shows the distribution of eggshell particles in the epichlorohydrin crosslinked chitosan matrix. The distribution of eggshell particles in the microstructure of the composites is the major factor responsible for the improvement of tensile strength of the composites. Incorporation of eggshell particles in crosslinked chitosan can lead to the production of low cost polymer composites.
Acknowledgements
The financial support of the Directorate General of Higher Education, Indonesia (DIKTI), is acknowledged.
References
- Sell, S. A.; Wolfe, P. S.; Garg, K.; McCool, J. M.; Rodriquez, I. A.; Bowlin, G. L. Polym. 2010, 2, 522-553
CrossRef - Goff, K. J. L.; Gaillard, C.; Helbert, W.; Garnier, C.; Aubry, T. Carbohydr. Polym.2015, 116, 117-123
CrossRef - Rahmi; Itagaki, H. J. Photopolym. Sci. Technol. 2011, 24(5), 517-521
- Khan, A.; Khan, R. A.; Salmieri, S.; Tien, C. L.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M. R. Carbohydr.Polym.2012, 90, 8, 1601-1608.
CrossRef - Motawie, A.M.; Mahmoud, K. F.; El-Sawy, A.A.; Kamal, H. M.; Hefni, H.; Ibrahiem, H.A. Egypt. J. Petrol. 2014, 23, 221-228.
CrossRef - Tirtom, V.N.; Dincer, A.;Bacerik, A., Aydemir, T.;Celik, A.Chem. Eng. J.2012,197(8), 379-386.
CrossRef - Suye, S. I.; Mizusawa, A.Se’i Gakkaishi1999, 55(2),73-77.
CrossRef - Laus, R.; Costa, T. G.;Szpoganicz, B.;Favere, V.T. J. Hazard. Mater, 2010, 183,233-241.
CrossRef - Rahmi;Fathurrahmi;Irwansyah; Purnaratrie, A.,Orient. J. Chem.2015, 31(4), 2071-2076.
- Rahmi, Asian J. Chem. 2016, 28(10), 2267-2271.
- Hassan, S. B.; Aigbodion, V.S. J.King Saud University-Engineering Sci. 2015, 27,49-56.
- Mohammadnezhad, J.; Soreshjani, F.K.; Bakshi, H. Desalination and Water Treatment, 2014, 1–12
- Ni, M.; Ratner B.D.Surf. Interface Anal.2008, 1-19.
- Paulino, A. T.; Santos, L. B.;Nozaki, J.React. Funct. Polym. 2008, 68,634-642.
CrossRef - Wang, J.; Becker, U. American Mineralogist, 2009, 94, 380-386.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









