Calcium Ion Selective Electrode Based on Schiff Base as Ionophore and Determination of Thermodynamic Functions and its Analytical Application
A. Vijayalakshmi and J. Thamarai selvi
Department of Chemistry, Avinashilingam Institute for Home Science and Higher Education for Women Coimbatore- 641043, Tamilnadu, India.
Corresponding Author E-mail: thamaraimuthu1973@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330163
A new, efficient Calcium ion selective electrode has been prepared using Schiff base based ionophore.The influence of temperature on electrode potential was studied and it can be used in the determination of thermodynamicfunction like ∆G,∆H&∆S.The electrochemical impedance spectroscopy (EIS) technique is also employed to study the electrochemical&surface reactions. It was also successfully used in the analysis of concentration of Calcium ion in various real samples.
KEYWORDS:Calcium (II); Schiff base; Potentiometry; Electrochemical impedance spectroscopy.
Download this article as:| Copy the following to cite this article: Vijayalakshmi A, selvi J. T. Calcium Ion Selective Electrode Based on Schiff Base as Ionophore and Determination of Thermodynamic Functions and its Analytical Application. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Vijayalakshmi A, selvi J. T. Calcium Ion Selective Electrode Based on Schiff Base as Ionophore and Determination of Thermodynamic Functions and its Analytical Application. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=30456 |
Introduction
The introduction of new ion-selective electrodes has played a fundamental role in the development of various sensory elements according to the charge and size of the target ion in clinical and environmental assays[1-8]. Potentiometric methods using ISEs for determining the metal ion have been studied extensively due to their importance in biological process[9,10],easy handling, nondestructive analysis and in expensive sample preparation, applicability to coloured sample and turbid solution.Calcium formation of bone,neuro muscular function,coagulation&membrane permeability.In plants it helps in transpiration which leads to growth of the plant.
Bedlechowicz et al;2002 developed calcium ion selective electrode using ETH1001 as an ionophore.In 2004 Kumar&Mittal developed a new Calcium ion selective electrode based on PVC membrane modified by a new ionophore dibenzo-18-crown-6(DB18C6).All solid state miniature Calcium ion selective electrode using Poly(3,4-ethylenedioxythiophene)doped with poly(styrenesulphonate)was carried out by Hui et al;2013.All solid -state Calcium ion selective electrode prepared of soluble electrically conducting polyaniline.(Lindfors,T and Ivaska, A, 2000).
Taking into consideration of all the above facts that a new simple ionophore such as Vinyl acetic acid grafted Pvc have been used as an electroactive phase for the fabrication of Ca2+ ion selective electrodes . In the present study the influence of temperature on electrode potential was studied&it can be used in the determination of thermodynamicfunction like ∆G,∆H&∆S.The electrochemical impedance spectroscopy(EIS)technique is also employed to study the electrochemical&surface reactions and the results are presented in this paper.
Experimental Method
Chemicals and Reagents used
Glycine , Reagent grade tetrapropyl ammonium bromide ,tetrahydrofuran,,Ethyl acetate,Dimethyl Acetamide,DMF, Dioctyl phthalate (DOP), sodium tetra phenyl borate (NATBP), tetra hydro furan (THF) were obtained from E.Merck and can be used without further purification. Throughout double distilled ionized water used.
Synthesis of Ionophore and Fabrication of the Electrode
For preparing Schiff base ionophore ,1.1ml of Salicylaldehyde solution was mixed with 0.75g of glycine&the mixture was stirred.11&this ionophore is used in the fabrication of the electrode.
Temperature Study
The influence of the temperature on electrode potential was studied by standard procedure (Gurtu and Gurtu, 2011)) for the prepared electrode in different calcium ion concentrations at various temperature ranges from 5 – 40°C. Because of the different temperatures, the ionic mobility of the ions which are to be analysed is different, so the potential was change.
Effect of temperature on electrode potential of the calcium ion selective electrode in the presence of aqueous, aquo-solvent and aqueous-buffer (pH) solution was used for the determination of thermodynamic functions like ∆G, ∆H and ∆S.
For the study of the effect of temperature on the output response, the temperature was changed in steps of 5°C. The values are tabulated in Table -1
Table 1: Effect of temperature on Ca- ISE
|
Medium |
Ca-Ion Selective Electrode |
(∂E/∂T)p v/K0 |
|||||||
|
EMF (volts) at different temperature |
|||||||||
|
278K |
283K | 288K | 293K | 298K |
303K |
308K |
313K |
||
| Aqueous |
0.199 |
0.216 | 0.218 | 0.220 | 0.228 |
0.230 |
0.238 |
0.250 |
0.0016 |
| Aqueous+ | |||||||||
| Solvent | |||||||||
| 25% of acetone |
0.197 |
0.203 | 0.207 | 0.211 | 0.214 |
0.219 |
0.223 |
0.228 |
0.0008 |
| 50% of acetone |
0.199 |
0.207 | 0.210 | 0.214 | 0.216 |
0.221 |
0.225 |
0.231 |
0.0009 |
| 75% of acetone |
0.203 |
0.210 | 0.213 | 0.216 | 0.219 |
0.223 |
0.227 |
0.235 |
0.0009 |
|
25% of ethanol |
0.196 |
0.201 |
0.204 |
0.209 |
0.213 |
0.219 |
0.221 |
0.225 |
0.0008 |
| 50% of ethanol |
0.198 |
0.204 | 0.207 | 0.212 | 0.216 |
0.221 |
0.224 |
0.227 |
0.0008 |
| 75% of ethanol |
0.201 |
0.209 | 0.211 | 0.215 | 0.219 |
0.224 |
0.229 |
0.230 |
0.0008 |
|
25% of DMA |
0.198 |
0.202 |
0.207 |
0.211 |
0.215 |
0.218 |
0.222 |
0.227 |
0.0014 |
| 50% of DMA |
0.202 |
0.206 | 0.211 | 0.213 | 0.216 |
0.220 |
0.223 |
0.228 |
0.0007 |
| 75% of DMA |
0.204 |
0.206 | 0.207 | 0.212 | 0.217 |
0.223 |
0.226 |
0.231 |
0.0007 |
|
25% of DMA |
0.199 |
0.203 |
0.205 |
0.209 |
0.214 |
0.219 |
0.221 |
0.226 |
0.0007 |
| 50% of DMA |
0.203 |
0.207 | 0.211 | 0.213 | 0.218 |
0.221 |
0.225 |
0.228 |
0.0007 |
| 75% of DMA |
0.206 |
0.209 | 0.212 | 0.216 | 0.219 |
0.223 |
0.227 |
0.231 |
0.0007 |
|
Aqueous+ |
|||||||||
| pH 3.43 |
0.155 |
0.167 | 0.181 | 0.190 | 0.205 |
0.210 |
0.216 |
0.225 |
0.0018 |
| pH 4.64 |
0.159 |
0.170 | 0.186 | 0.193 | 0.208 |
0.213 |
0.221 |
0.228 |
0.0018 |
| pH 5.54 |
0.162 |
0.173 | 0.188 | 0.195 | 0.209 |
0.215 |
0.225 |
0.229 |
0.0020 |
| pH 6.24 |
0.165 |
0.179 | 0.192 | 0.201 | 0.210 |
0.219 |
0.229 |
0.230 |
0.0019 |
The E.M.F. values of calcium ion selective electrode using the cell, Ca, Ca ionophore // Ag/AgCl was measured at different temperature ranging from 5 – 40°C. The relationship between T(∂E/∂T)p and E of calcium ion selective electrode in different media was linear. The relationship was linear in all the cases in accordance with the equation
E =-∆H/nF+T(∂E/∂T)p
The value of temperature co-efficient (∂E/∂T)p in Table-1 in aqueous, aqueous – solvent, aqueous – buffer solution have been used in the calculation of ∆G, ∆H and ∆S at 5-40°C for the calcium ion selective electrode and the results were presented in the Table -2
Table 2: Thermodynamic parameter values of ∆G, ∆H and ∆S
| Medium | -∆G K.cal/mole | ∆H K.cal/mole | ∆S e.u |
| Aqueous | 43.4009 | 7.4835 | 178.525 |
| Aqueous + | |||
| solvent | 41.0586 | 8.7594 | 168.87 |
| 25% of acetone | 41.5638 | 9.6522 | 173.70 |
| 50% of acetone | 42.1173 | 9.3065 | 173.60 |
| 75% of acetone | |||
| 40.7230 | 4.7815 | 154.40 | |
| 25% of ethanol | 41.2296 | 4.7236 | 159.22 |
| 50% of ethanol | 41.8328 | 3.4547 | 154.40 |
| 75% of ethanol | |||
| 41.0125 | 4.5162 | 154.40 | |
| 25% of DMA | 41.4709 | 1.3510 | 144.75 |
| 50% of DMA | 41.6398 | 1.4067 | 144.75 |
| 75% of DMA | |||
| 40.9138 | 1.8846 | 144.75 | |
| 25% of DMF | 41.6350 | 4.2305 | 135.10 |
| 50% of DMF | 42.0455 | 0.8450 | 144.75 |
| 75% of DMF | |||
| Aqueous+ | |||
| pH 3.43 | 37.3675 | 68.430 | 361.875 |
| pH 4.64 | 38.0643 | 67.8075 | 361.875 |
| pH-5.57 | 38.5035 | 79.3181 | 400.475 |
| pH- 6.24 | 39.1994 | 68.145 | 366.70 |
Electrochemical Study
The impedance spectrum of the electrode was recorded at 10 mV amplitude in the frequency range of 0.01 to 1000 Hz. Zm
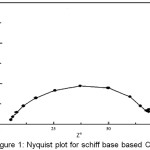 |
Figure 1: Nyquist plot for schiff base based Ca-ISE
|
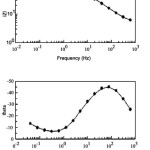 |
Figure 2: Bode plot for schiff base based Ca-ISE
|
 |
Figure 3: Calcium ion selective electrode based on schiff base ionophore
|
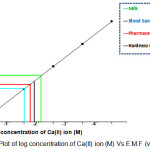 |
Figure 4: Plot of log concentration of Ca(II) ion (M) Vs E.M.F (volts) Click here to View Figure |
Conclusion
A new simple ,highly specific&selective calcium ion electrode has been prepared.The life time of the prepared electrode was found to be 3 months with good reproducibility of E.M.F values.The thermodynamic parameter value∆ G,∆H,∆S of the electrode has successfully determined.
Reference
- Shamsipur.M ,Soleymanpour.A , Akhond.M, Sharghi.H and Massah.A.R “Uranyl selective PVC membrane electrodes based on some recently synthesized benzo-substituted macrocyclic diamides,” Talanta, 2002. 58, (2); 237–246,
CrossRef - Zhang Z.Rand Yu.R.Q, “The synthesis and membrane transport characteristics of macrocyclic polyether ligands composed of 1,10-phenanthroline as carriers for primary amine species,” Talanta,1994, 41, (2); 327–333,
CrossRef - Singh.A.K, Saxena.P, Mehtab.P and.B Gupta, “Strontium(II)- selective electrode based on a macrocyclic tetraamide,” Talanta,2006, 69, (2); 521–526,
CrossRef - Singh,A.K, Saxena,P.and Panwar,A “Manganese(II)-selective PVC membrane electrode based on a pentaazamacrocyclic manganese complex,” Sensors and Actuators B, 2005, 110, (2); 377-381.
CrossRef - Ganjali,M.R, Razavi,T , Dinarvand,R . Riahi.S and Norouzi,P “New diltiazem potentiometric membrane sensor stands on theoretical calculations as a useful device for diltiazem hydrochloride analysis in pharmaceutical formulation and urine,” International Journal of Electrochemical Science,2008, (3); 1543–1558,
- Ganjali.M.R Memari.Z, Faridbod.F and Norouzi.P “Samarium microsensor: an asymetric potentiometric membrane sensor,” International Journal of Electrochemical Science,2008, 3, 1169-1179.
- Beheshti,S.S and Amini.M.K “A simple and selective flow injection potentiometric method for determination of iodide based on a coated glassy carbon electrode sensor,” International Journal of Electrochemical Science,2007, 2,778–787,
- Zhang.H Zhang.Z Li.J and Sh. Cai, “Effects of Mg2+ on supported bilayer lipid membrane on a glassy carbon electrode during membrane formation,” International Journal of Electrochemical Science,2007, 2, 788–796,
- Paker.P, McGraw-Hill Concise Encyclopaedia of Science and Technology, McGraw-Hill, New York, NY, USA, 1994,
- D’Souza.S.F, “Microbial biosensors,” Biosensors and Bioelectronics,2001,16, (6), 337–353.
- Vijayalakshmi.A,Thamarai selvi,J,Calcium ion selective electrode based on Schiff base as an electroactive material-Its preparation and analytical applications,International journal of current research,2013,5,(8):2176-2178.
- Wang,Y;Xu,H;Yang,X;Luo,Zhang,J.andLi,G;All solid-state blood calcium sensors based on screen-printed poly(3,4-ethylenedioxythiophene)as the solid contact,Sensors and Actuators B, 2012, 173: 630-635.
CrossRef - Rafel Hernandez,Jordi Riu and Xavier Rius,F;,Determination of calcium ion in sap using carbon nano tube-based ion selective electrodes,Analyst ,2010, 135: 1979-1985.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









