Chemical studies on the preparation of magnetic nanoparticles coated with glycine and its application for removal of heavy metals
Jawaher Alzaidi, Eman Alzahrani and N. R. A. El-Mouhty
Chemistry Department, Faculty of Science, Taif University, 888-Taif, Kingdom of Saudi Arabia Corresponding Author Email: em-s-z@hotmail.com
DOI : http://dx.doi.org/10.13005/ojc/320324
Article Received on : May 22, 2016
Article Accepted on : June 30, 2016
Article Published : 28 Jun 2016
The aim of this study is the preparation of magnetic nanoparticles and coating with glycine to remove heavy metals such as Cu+2. The magnetic nanoparticles were prepared by co-precipitation method using using ferrous sulphate and potassium nitrate in presence of potassium hydroxide. Different instrumental analysis such as XRD, TEM, SEM and EDAX were used to study the magnetic nanoparticles which produced and comparing it after coated with glycine. The optimum conditions which reflect the high efficiency of removal are pH 10, concentration of the heavy metal 200 ppm, dosage 0.05 g and for 24 h duration time. Therefore we recommend using magnetic nanoparticles coated with glycine for removal of heavy metals.
KEYWORDS:Magnetic nanoparticles; glycine; X-ray diffraction; Fe3O4; co-precipitation; heavy metals
Download this article as:| Copy the following to cite this article: Alzaidi J, Alzahrani E, El-Mouhty N. R. A. Chemical studies on the preparation of magnetic nanoparticles coated with glycine and its application for removal of heavy metals. Orient J Chem 2016;32(3) |
| Copy the following to cite this URL: Alzaidi J, Alzahrani E, El-Mouhty N. R. A. Chemical studies on the preparation of magnetic nanoparticles coated with glycine and its application for removal of heavy metals. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=18209 |
Introduction
With the rapid development of nanotechnology, magnetic nanoparticles are currently studied widely. magnetic nanoparticles have attracted much attention because of their unique magnetic properties, small size, low toxicity, and widespread application, Iron oxide NPs are one of the most common NPs that could find their way into the water system because of their use in water and soil treatment [1].
Nanoparticles are good building blocks for developing high-capacity sorbents with modification ability to enhance their affinity and selectivity for purification of contaminated waters. Application of nanoparticles (NPs) as novel adsorbents for removal of pollutants is gaining research interest[2].
Co-precipitation is the most conventional method and is also used by several commercial production plants for the synthesis of magnetic or black iron oxides. there is problem associated with particles in this size range is their intrinsic instability over longer periods of time. Such small particles tend to form agglomerates to reduce the energy associated with the high surface area to volume ratio of the nanosized particles[3].
Moreover, naked metallic nanoparticles are chemically highly active, and are easily oxidized in air, resulting generally in loss of magnetism and dispersibility, coating helps not only in protecting the core shell but is also used in addition of functional groups for specific pollutant removal in water treatment[4].
Generally, magnetic NPs are surface modified with carboxyl, hydroxyl and amino groups for their specific interactions [5], The synthesis of magnetite nanoparticles has been intensively developed not only for its fundamental scientific interest but also for many technological applications, the use of magnetite nanoparticles as adsorbents in water treatment provides a convenient approach for separating and removing the contaminants by applying external magnetic fields. Bare magnetite nanoparticles are susceptible to air oxidation and are easily aggregated in aqueous systems [6]. Thus, for the application of these nanoparticles in various potential fields the stabilization of the iron oxide particles by surface modification is desirable. The magnetic structure of the surface layer, which is usually greatly different from that in the core of the nanoparticles, can have a notable effect on the magnetic properties of nanoparticles [7].
Experimental
Chemicals and materials
Iron (II) sulphate heptahydrate, ferrous sulphate (FeSO4.7H2O), M.wt=278.01g mol-1 (98%), potassium nitrate (KNO3) M.wt=101.1032 g mol-1, potassium hydroxide (KOH) M.wt=56.11 g mol-1, were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China), Glycine (NH 2CH 2COOH), sodium hydroxide (NaOH), hydrochloric acid (HCl), copper Sulphate (CuSO4), were purchased from BDH Chemicals Ltd. (Poole, UK) and used without further purification. Cylindrical rod magnets (40 mm diameter x 40 mm thick) for settlement of magnetic nanoparticles was purchased from Magnet Expert Ltd. (Tuxford, UK). Distilled water was employed for preparing all the solutions and reagents.
Instrumentation
Magnetic stirrer and heater from Fisher Scintifi Co Ltd (Shanghai, China). Oven from Memmert (Nuremberg, Germany). PH meter from Hanna Instruments (Cluj, Romania). Balance from Mettler Toledo Model JB1603c/FACT (Greifensee, Switzerland). Sonicator UltraSonic bath from wiseclean Model WUC-D10H (Wonju, Korea). UV-VIS Spectroscopy Thermo Fisher Scientific, GENESYS 10S VIS (Madison, USA). X-ray diffraction patterns (XRD ) were obtained using Bruker diffractometer D8-advance CuKα1-radiation (Coventry, UK). The transmission electron microscopy (TEM) came from JEOL Ltd. (Welwyn Garden City, UK).Scanning Electron Microscope (SEM ) with unit Energy Dispersive X ray (EDAX) analysis, JEOL JSM 6390 LA Analytical(Tokyo, Japan).
Preparation of magnetic nanoparticles MNPs
Magnetic nanoparticles were prepared as described else where by Abu Bakar et al.[2]. 1.67g of FeSO4.7H2O, dissolved in 50 mL of distilled water, 1.01 g of KNO3 dissolved in 10 mL of distilled water, and 2.5 M of KOH were prepared. Potassium nitrate solution was added to FeSO4.7H2O solution and stirring was continued for 30 min. Then, 10 mL of KOH solution (2.5 M) was slowly added to the above solution. The reaction mixture was heated to 100 °C and maintained at this temperature for 2 h. The mixture was cooled down to room temperature, and the black precipitate was repeatedly washed with distilled water, and allowed to dry at 50 °C over night. The weight of the prepared MNPs using both techniques was measured.
Coating of MNPs with glycine
One gram of glycine was dissolved in 50 mL of distilled water. Then, 1 g of the prepared MNPs was added to the previous solution. The mixture was sonicated for 2 h. After that the magnetic nanoparticles coated with glycine was washed with distilled water and they were dried at room temperatures for 24 h.
Characterization of the prepared materials
Characterizations of the fabricated bare MNPs and MNPs-Gly were carried out using X-ray diffractometer (XRD) to determine the crystalline structure of the particles. The transmission electron microscopy (TEM) was used for size investigation, scanning electron microscopy (SEM) for surface morphology analysis, energy dispersive X-ray analysis (EDAX) to the compositional analysis.
Removal of heavy metals
The optimum condition for removal of heavy metal Cu+2 ions by the magnetic nanoparticles was investigated in aqueous solution at 25◦C, chemical parameters were studied as the following
Effect of different pH of solution
We studied the effect of different pH (2, 4, 6, 8, 10), where the mixture was adjusted to certain pH with NaOH or HCl and mixed by ultrasonication several minutes. using 10 ml of solution that contain Cu+2 ions, its concentration 800 ppm, separately and with 0.001 g of MNP, on 700 rpm magnetic stirrer, for 1 h.
Effect of different concentrations of heavy metals
We studied the effect of different concentrations (200, 400, 600, 800, 1000 ppm), at optimum pH that we studied, using 0.001 g of MNP, for 1 h.
Effect of different dosages of MNPs and Gly-MNPs
We studied the effect of different dosages of magnetic nanoparticles (0.001, 0.002, 0.005, 0.01, 0.05 g), at optimum pH and concentration that we studied.
Effect of different contact time
We studied the effect of different contact time (30, 60, 120, 180 and 24 h), at optimum pH ,concentration and dosage that we studied.
Results and Discussion
Preparation of magnetic nanoparticles (MNPs)
The aim of this study was to fabricate magnetic nanoparticles in order to use them for removal of heavy metals. The reasons for choosing this types of nanoparticles are because they are easy to separated from the solution by using external magnet, and easy to fabricated . In this study, two different methods were ulilised to prepare MNPs, which will be discussed in the following see there Figure 3.6 shows the prepared magnetic nanoparticles using second method in solution (A), and after separation using external magnet. Figure 3.6 (C) shows the dried magnetic nanoparticles. The prepared magnetic nanoparticles were characterized using different techniques which will be discussed in the following
FeSO4.7H2O + KOH + KNO3→Fe3O4 (s) + K2SO4 + H2O
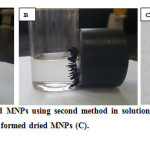 |
Fig. 3.1: shows the prepared MNPs using second method in solution(A), after separation using external magnet, and (B) the formed dried MNPs (C). Click here to View figure |
TEM micrographs of the prepared Fe3O4 nanoparticles are shown in Figure 3.1. The TEM images reveal that Fe3O4 nanoparticles are similar in shape and appear to be uniformly dispersed and had cube shape. It clearly showed that they have distinctive outlines square.
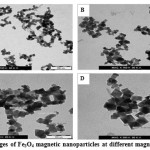 |
Figure 3.2: TEM images of Fe3O4 magnetic nanoparticles at different magnifications. Click here to View figure |
Surface morphology of Fe3O4 was studied using SEM analysis, as can be seen in Figure 3.2. that shows cube shaped crystals structure of Fe3O4.can find that most of the particles are roughly cube and aggregate together because of their high surface energy and adhesion.
 |
Figure 3.3: SEM analysis of Fe3O4 magnetic nanoparticle at different magnifications. Click here to View figure |
Figure 3.3 show The EDAX spectrum that shows iron and oxygen. It reveals that the iron was from magnetic Fe3O4. The elements of Fe and O can be observed in the EDAX pattern, These results demonstrate the purity of the synthesis results. Table 3.4 shows the composition components of the formed Fe3O4 where the percentage of mass of O is 40.64%, and Fe is 59.36%, while the atom percentage of O is 70.50%, and Fe is 29.50%.
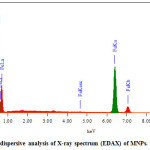 |
Figure 3.4: Energy dispersive analysis of X-ray spectrum (EDAX) of MNPs. Click here to View figure |
Table 3.1. The composition components of the formed Fe3O4 using EDAX analysis.
|
Element |
Mass% |
Atom% |
|
O K |
40.64 |
70.50 |
|
Fe K |
59.36 |
29.50 |
|
Total |
100.00 |
100.00 |
XRD analysis
Figure 3.4 shows the XRD spectrum of the prepared magnetic nanoparticles using second method, XRD pattern of the prepared Fe3O4 using second method shows six characteristic peaks at 30o, 35o, 43o, 53o, 57o, and 62o were corresponding to the (220), (311), (400), (422), (511), and (440) crystal planes of a pure Fe3O4 with a spinal structure. The peaks indicating that Fe3O4 with a spinal structure and no characteristic peak of impurities are detected in the XRD pattern[8]. The XRD results also confirmed the purity of the products via the absence of other phases of iron oxide such as maghemite or hematite in sample
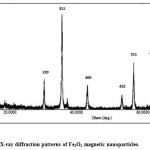 |
Figure 3.5: X-ray diffraction patterns of Fe3O4 magnetic nanoparticles. Click here to View figure |
Coating of magnetic nanoparticles MNPs with glycine
Surface modification of magnetic nanoparticles is a challenged key for different applications and can be accomplished by physical/ chemical adsorption of organic compounds[9].
Coating of magnetic nanoparticles can decrease the toxicity of MNPs through decreases the saturation magnetization and increases the particle volume. It is important to consider that smallest particles present highest adsorption capabilities and high magnetizations optimize the magnetic separation. To avoid these constraints, an alternative to polymer coatings is the chemical modification of the magnetic nanoparticle surface by small functional molecules. In this way, easily adsorbed onto the iron oxide surface, and NH2 groups at surface that could interact with the bio-environment. In fact, the potential of amine functionalized magnetic nanoparticles was already evidenced for magnetic removal of organic contaminants and heavy metals in water treatment[10].
TEM analysis
TEM measurements showed that synthesis yields nearly cubic Gly-MNPs and regular in shape, and close in size dramatically. Moreover, there is no significant difference in shape of functionalized nanoparticles and non-functionalized ones, as showed in Figure 3.9.
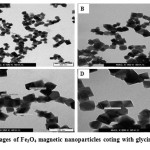 |
Figure 3.6: TEM images of Fe3O4 magnetic nanoparticles coting with glycin. Click here to View figure |
SEM-EDAX analysis
Figure 3.10. image scanning electron microscope clearly shows a layer of glycine encapsulate nanoparticles compared to the previous image in Figure 3.6. where completely covered show and this proves the success of the coating of magnetic nanoparticles.
 |
Figure 3.7: SEM analysis of Fe3O4 magnetic nanoparticle coating with glycin. Click here to View figure |
The EDAX spectrum of Gly-MNPs sample is shown in Figure 3.11, and it clearly shows the evidence of Fe, O and C. This result presented solid proof of the proposed structure of Gly-MNPs.
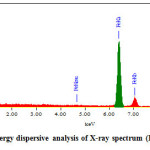 |
Figure 3.8: Energy dispersive analysis of X-ray spectrum (EDAX) of Gly-MNPs. Click here to View figure |
Table 3.2: The composition components of the formed Fe3O4
|
Element |
Mass(%) |
Atom(%) |
|
C K |
17.63 |
33.91 |
|
O K |
31.06 |
44.86 |
|
Fe K |
51.31 |
21.23 |
|
Total |
100.00 |
100.00 |
XRD analysis
Fig.3.12. shows XRD pattern of Fe3O4 MNPs coated with glycine. The presence of six characteristic sharp and intense peaks confirmed the formation of highly crystalline nanoparticles. And was found there on difference with XRD result of second method.
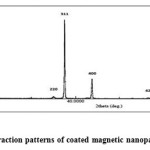 |
Figure 3.9: X-ray diffraction patterns of coated magnetic nanoparticles. Click here to View figure |
The impact of various factors on adsorption of heavy metal ion
![]()
Where: C0 and C are the initial and residual concentrations of the heavy metal in the solution (ppm), respectively.
To obtain the optimum condition for removal of heavy metal different chemical parameters were studied as the following:
- Effect of different pH of solution.
- Effect of different concentration of heavy metals.
- Effect of different dosage of MNPs and Gly-MNPs
- Effect of different contact time.
Effect of different pH of solution
Table 3.3: Effect of different pH on the removal of Cu+2using Gly-MNPs
| %Up take of Gly- MNPs | %Up take of MNPs | PH |
| 50 | 41.25 | 2 |
| 65 | 63 | 4 |
| 85 | 75.1 | 6 |
| 97.5 | 80 | 8 |
| 98 | 89 | 10 |
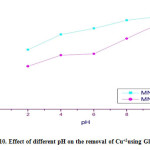 |
Figure 3.10: Effect of different pH on the removal of Cu+2using Gly-MNPs Click here to View figure |
As shown in tables no. 3.3. and Figures 3.10. it can be concluded that heavy metal adsorbed on the Gly-MNPs increased with pH value where the %up take was 100% at pH 10.
This observation may by due to electrostatic attraction between the negatively charged, Gly-MNPs surface and the positively charged heavy metals Cu+2[11].
Table 3.4: Effect of different concentrations on the removal of Cu+2using Gly-MNPs
| %Up take of Gly- MNPs | %Up take of MNPs | Conc. (ppm) |
| 100 | 88 | 200 |
| 100 | 82.5 | 400 |
| 100 | 78 | 600 |
| 100 | 65 | 800 |
| 87 | 63 | 1000 |
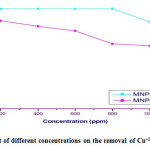 |
Figure 3.11: Effect of different concentrations on the removal of Cu+2using Gly-MNPs Click here to View figure |
According to Table no. 3.4. and Figure no. 3.11. we can be note that the percentage for removal heavy metal decreases from 100% to 87% with increasing concentration of heavy metal for (200-1000 ppm). At equilibrium unit adsorption increased from (200 to 600 ppm), and after that the %up take decreased, this is because the mass transfer driving force would become larger as the initial concentration increased. Hence, it results in higher heavy metals adsorption[12].
Effect of the dosages of MNPs and Gly-MNPs
Table 3.5: Effect of different dosages on the removal of Cu+2 using Gly-MNPs
| %Up take of Gly- MNPs | %Up take of MNPs | Dose (g) |
| 62.4 | 46.66 | 0.001 |
| 77.9 | 54.6 | 0.002 |
| 86.8 | 64 | 0.005 |
| 93 | 71.7 | 0.01 |
| 100 | 88 | 0.05 |
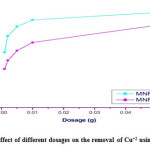 |
Figure 3.12: Effect of different dosages on the removal of Cu+2 using Gly-MNPs Click here to View figure |
Table no. 3.5 and Figure no. 3.12 represent that the highest percent of removal of Cu+2 increased from (62.4 to 100 %) as the adsorbed dosage increased. Where as the adsorbent dosage increases the surface area of the adsorbent will be increased. Hence, more adsorption sites are available to adsorb heavy metals from aqueous solution[13].
Effect of the contact time
Table 3.6: Effect of contact time on the removal of Cu+2 using Gly-MNPs.
| %Up take of Gly- MNPs | %Up take of MNPs | Time (h) |
| 76.66 | 26.66 | 0.5 |
| 93.3 | 35 | 1 |
| 98.3 | 56 | 2 |
| 100 | 78 | 3 |
| 100 | 81 | 24 |
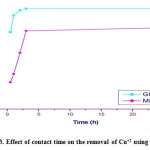 |
Figure 3.13: Effect of contact time on the removal of Cu+2 using Gly-MNPs. Click here to View figure |
From Table no. 3.6, and Figure no. 3.13 the trend of the plots show the system with take longer contact time to achieve adsorption equilibrium.
The Gly-MNPs first has to reach the boundary layer and them diffuses into the tiny pores of the adsorbent during the adsorption process.This phenomenon takes relatively longer contact time[14].
Conclusions
Advances in nanoscale science and engineering are providing new opportunities to develop technology. The experimental results showed that MNPs were prepared by co-precipitation methods and different chemical analysis indicating that the MNPs coated with glycine. This study had shown that the difference between of Gly-MNPs for removal of heavy metals compared with naked MNPs. The obtain Gly-MNPs was used as adsorbents for removal of heavy metals. where magnetic nanoparticles contain high surface area and large pore size for heavy metal to be adsorbed on the surface of Gly-MNPs.
References
- G.-y. Li, Y.-r. Jiang, K.-l. Huang, P. Ding, and J. Chen, “Preparation and properties of magnetic Fe 3 O 4–chitosan nanoparticles,” Journal of alloys and compounds, vol. 466, pp. 451-456, 2008.
CrossRef - H. Parham, B. Zargar, and R. Shiralipour, “Fast and efficient removal of mercury from water samples using magnetic iron oxide nanoparticles modified with 2-mercaptobenzothiazole,” Journal of hazardous materials, vol. 205, pp. 94-100, 2012.
CrossRef - S. A. Jadhav and R. Bongiovanni, “Synthesis and organic functionalization approaches for magnetite (Fe3O4) nanoparticles,” Adv Mat Lett, vol. 3, pp. 356-361, 2012.
CrossRef - Y. Ju-Nam and J. R. Lead, “Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications,” Science of the total environment, vol. 400, pp. 396-414, 2008.
CrossRef - M. Mahmoudi, S. Sant, B. Wang, S. Laurent, and T. Sen, “Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy,” Advanced drug delivery reviews, vol. 63, pp. 24-46, 2011.
CrossRef - D. Maity and D. Agrawal, “Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media,” Journal of Magnetism and Magnetic Materials, vol. 308, pp. 46-55, 2007.
CrossRef - L. Zhang, R. He, and H.-C. Gu, “Oleic acid coating on the monodisperse magnetite nanoparticles,” Applied Surface Science, vol. 253, pp. 2611-2617, 2006.
CrossRef - P. Loekitowati Hariani, M. Faizal, R. Ridwan, M. Marsi, and D. Setiabudidaya, “Synthesis and properties of Fe3O4 nanoparticles by co-precipitation method to removal procion dye,” International Journal of Environmental Science and Development, vol. 4, pp. 336-340, 2013.
CrossRef - M. Takafuji, S. Ide, H. Ihara, and Z. Xu, “Preparation of poly (1-vinylimidazole)-grafted magnetic nanoparticles and their application for removal of metal ions,” Chemistry of materials, vol. 16, pp. 1977-1983, 2004.
CrossRef - N. C. Feitoza, T. D. Goncalves, J. J. Mesquita, J. S. Menegucci, M.-K. M. Santos, J. A. Chaker, et al., “Fabrication of glycine-functionalized maghemite nanoparticles for magnetic removal of copper from wastewater,” Journal of hazardous materials, vol. 264, pp. 153-160, 2014.
CrossRef - S. S. Banerjee and D.-H. Chen, “Fast removal of copper ions by gum arabic modified magnetic nano-adsorbent,” Journal of hazardous materials, vol. 147, pp. 792-799, 2007.
CrossRef - J. Porath and B. Olin, “Immobilized metal affinity adsorption and immobilized metal affinity chromatography of biomaterials. Serum protein affinities for gel-immobilized iron and nickel ions,” Biochemistry, vol. 22, pp. 1621-1630, 1983.
CrossRef - Y. Shen, J. Tang, Z. Nie, Y. Wang, Y. Ren, and L. Zuo, “Preparation and application of magnetic Fe 3 O 4 nanoparticles for wastewater purification,” Separation and Purification Technology, vol. 68, pp. 312-319, 2009.
CrossRef - J.-L. Gong, X.-Y. Wang, G.-M. Zeng, L. Chen, J.-H. Deng, X.-R. Zhang, et al., “Copper (II) removal by pectin–iron oxide magnetic nanocomposite adsorbent,” Chemical Engineering Journal, vol. 185, pp. 100-107, 2012.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









