Cationic Ring Opening Copolymerization of 1,3-Dioxolane with Styrene by Montmorillonite Maghnite-H+Catalyst
Nabil Hamam*, Mohammed Issam Ferrahi and Mohammed Belbachir
Polymer Chemistry Laboratory, Department of Chemistry, Faculty of Science, University of Oran 1 Ahmed Benbella BP N ° 1524 El M'Naouar, 31000 Oran, Algeria .
Corresponding Author E-mail: nabilpolymere@hotmail.fr
DOI : http://dx.doi.org/10.13005/ojc/320305
Article Received on : August 18, 2015
Article Accepted on : September 28, 2015
In the present work, the copolymerization of 1,3-Dioxolane (DXL) with Styrene (St) catalyzed by Maghnite-H+ a montmorillonite sheet silicate clay exchanged with protons, was investigated. The cationic ring opening polymerization was initiated by Maghnite-H+ in bulk. The copolymer obtained was characterized by 1H-NMR, DSC and IR spectroscopy. The studies done, such as the effect of the amount of catalyser on the syntheses of poly (DXL -co- Styrene).
KEYWORDS:Copolymerization; Montmorillonite Maghnite-H+ Catalyst; DXL
Download this article as:| Copy the following to cite this article: Hamam N, Ferrahi M. I, Belbachir M. Cationic Ring Opening Copolymerization of 1,3-Dioxolane with Styrene by Montmorillonite Maghnite-H+ Catalyst. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Hamam N, Ferrahi M. I, Belbachir M. Cationic Ring Opening Copolymerization of 1,3-Dioxolane with Styrene by Montmorillonite Maghnite-H+ Catalyst. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=17957 |
Introduction
Clay minerals, a large family of alumino-silicate structures with a range of chemical composition, structure and surface properties, are very reactive materials due to their small grain size large surface area, adsorption properties and chemical variability 1 . The reactions catalyzed by Clay minerals montmorillonite are usually carried out under mild conditions with high yields and high selectivity, and the workup of these reactions is very simple; only filtration to remove the catalyst and evaporation of the solvent are required. Montmorillonite catalysts are easily recovered and reused 2.
Increasing environmental concerns in recent years have resulted in a demand for more effective catalytic processes. In this regard, studies have been carried out on the development of solid acids, such as “Keggin-type Heteropolycompounds” 3 or acidic clays4, to replace aggressive and dangerous homogeneous acids to overcome the problems of separating the catalyst from the products and the disposal of solid/liquid wastes.
Recently, monomers were found to intercalate into lattice layers of montmorillonite clay, permitting in situ polymerizations yielding polymer/clay nano- composites. Several polymers, for example, polystyrene (PS)5, and polystyrene-block-polyisoprene-block-polystyrene6 ,an Algerian proton exchanged montmorillonite clay called Maghnite-H+ (Mag-H+), a new nontoxic cationic initiator, was used as a catalyst for cationic polymerization of a number of vinylic and heterocyclic monomers 7-8.
The purpose of this paper is to study the copolymerization of DXL with Styrene, catalyzed by Maghnite-H+ a proton exchanged Montmorillonite clay7. The effects of the amounts of the Maghnite-H+ on the synthesis of poly (DXL-co-Styrene) are also discussed.
Techniques such as Infra Red (IR), Differen- tial Scanning Calorimetry (DSC), Hydrogen and Proton Nuclear Magnetic Resonance (1H NMR), were used to characterize the products of the reaction.
 |
Figure 1: Synthesis of poly (DXL -co- Styrene) by Maghnite-H+ catalyst Click here to View Figure |
Experimental
Materials
Styrene (grade 99%) was used as purchased from Aldrich. 1,3-Dioxolane (DXL) was distilled over the blue benzophenone–Na complex.Chloroform was dried on CaH2 anhydrous and distilled before use. Raw-Maghnite: Algerian Montmorillonite clay was procured from BENTAL (Algerian Society of Bentonite).
Preparation of “Maghnite-H+ 0.25M”
Maghnite-H+ was prepared according to the process reported in our previous study8. Raw-Maghnite (20 g) was crushed for 20 mn using a prolabo ceramic balls grinder. It was then dried for 2 hours at 105◦C the Maghnite was placed in an Erlenmeyer flask together with 500 ml of distilled water. The Maghnite/water mixture was stirred using a magnetic stirrer and combined with 0.25 M sulfuric acid solution, until saturation was achieved over 2 days at room temperature, the mineral was then washed with distilled water to became sulfate free and then dried at 105◦C, a test of the barium nitrate of the residue of rinsing water is needed to ensure that the sulfate is eliminated.
Copolymerization and products characterization
In a 50 ml beaker, DXL (11.7 mol/l) and Styrene (6.3 mol/l) were dissolved in 20 ml of Chloroform and a chosen amount of Maghnite-H+ was added at 40◦C. The weight ratio was kept constant in all flasks. After the required time was reached, an aliquot of the reaction mixture was then removed in such a manner as to exclude any clay mineral, and then dried by evaporation to remove solvent and remaining monomer.
Results and Discussions
The results of experiments of DXL with Styrene Copolymerization induced by Maghnite-H+ 0.25M proceed in bulk are reported in (Table 1) . For all these experiments the temperature was kept constant at 40°C for 12 hours.
Table 1: Copolymerizations of 1,3-Dioxolane with styrene using different amount of Maghnite-H+
|
Experiment |
Maghnite-H+ (0.25% M) | Yield % |
| 1 | 10 | 39.56 |
| 2 |
5.0 |
19.23 |
| 3 | 2.5 |
10.13 |
Effect of the amount of Maghnite-H+ on the copolymerization
We can see from (Table 1) that the yield increases as the proportion of Maghnite-H+ 0.25 M increases (experiments 1,2). Shows the effect of the amount of Maghnite-H+ on the polymerization yield. Indeed, using various amounts: 2.5 , 5 and 10% by weight, the polymerization was carried out in bulk at 40◦C. The copolymerization yield increased with the amount, thus clearly showing the effect of Maghnite-H+ as a catalyst. This phenomenon is probably the result of an increase in the number of “initiating active sites” responsible of inducing polymerization, a number that is pro rata to the amount of catalyst used in reaction.
Characterization of products
According to the work published by Masahiko Okada10 the polymer obtained was characterized by 300 MHz 1H-NMR in CDCl3. (Figure 2) shows the chemical shifts at 7.23 ppm and 7.33 ppm for the protons of benzene ring, that at 3.74 ppm for the protons of methylene groups and that at 4.8 ppm for the protons of ethylene group of Poly DXL and those at 1.26 ppm and 1.74 ppm for the methylene and methine groups of Poly St.
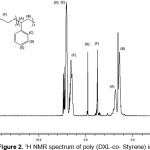 |
Figure 2:1H NMR spectrum of poly (DXL-co- Styrene) in CDCl3 Click here to View Figure |
IR spectroscopy (Figure 3) allows us to confirm the nature of the polymer by identifying, The phenyl of St appears in three absorption bands: one at 1645.77 cm-1 for the (C = C), another at 3157.29 cm-1 for (CH) and the last at 781.10 cm-1 for the deformation in the plan of (CH).The ether function appears clearly at 1051.42 cm-1 .The large band at 3473.29 cm-1 is attributed to the connection (OH), which is probably due to a bad drying of KBr used for this analysis.
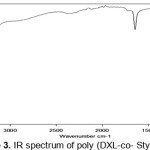 |
Figure 3: IR spectrum of poly (DXL-co- Styrene) Click here to View Figure |
The study of polymers degradation (Figure 4) can most often intervene on the factors which improve their thermal stability and also which allow to situate better their own domain of application.DSC analysis of the polymer to give a glass transition temperature obtained at 55.7 ◦C and a melting temperature at 141.1 ◦C, the presence of both the transition temperature and the melting temperature, shows that the polymer is semi crystalline.
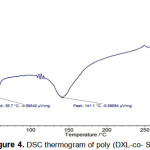 |
Figure 4: DSC thermogram of poly (DXL-co- Styrene) |
Conclusions
In conclusion, we have found that acid-exchanged Maghnite-H+ is effective as acidic catalyst for the preparation of poly (DXL -co- Styrene). The studies done such as the effect of the amount of catalyst on the syntheses of polymer. The polymerization proceeds smoothly by a very simple procedure, and a simple filtration is sufficient to recover the catalyst.
References
- Caglar, B. ; Afsin,B . ; Tabak, E.;Eren,E. Chemical. Engineering .Journal. 2009, 149,242-248.
CrossRef - Brown, D.R.; Carpathica, G. Clays as catalyst and reagent supports. Ser.Clays .1994, 45, 45.
- Wang, P.; Jia, Z.;Zhao, M.;Sun, Z.; Zhang, H.; Ji, S. ; Zhao, Y. J. Chem. Ind. Eng. Soc. China.2009,7: 12.
- Donald, R.; John, F. US Patent.1993, 5262562.
- Vu Moc, T.; Petit, H . ; Maitte, P. Bull. Soc. Belg. 1982, 91, 261.
- Vu Moc, T.; Petit, H . ; Maitte, P. Bull. Soc. Belg. 1982, 91, 261.
- Ferrahi, M.I. ; Belbachir, M. Int. J. Mol. Sci, 2003, 4, 312.
CrossRef - Belbachir, M.; Bensaoula, A. US Patent.2001,6, 274,527B1.
- Yahyaoui, A.; Belbachir, M. ; Hachemaoui,A. ,Int. J. Mol. Sci. 2003, 4, 572
CrossRef - Okada,M.; Yamashita,Y.; Ishii,Y. Die. Makromolekulare .Chemie,1966, 94, 1,181–193.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









