A Comparison Between Biocompatibilities of Nanocomposites of Silica Doped in HA/Collagen and Those Doped in HA/Gelatin
Fariba Najafizadeh1, Mir Abdullah Seyed Sadjadi1, Seyed Jemiladine Fatemi2, Mahmood Karimi Mobarakeh3, and Reza Malekpour Afshar4
1Department of Inorganic Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran 2Department of Chemistry, Basic Sciences Faculty, Islamic Azad and Shahid Bahonar University, Kerman, Iran 3Department of Trauma and Orthopedic Surgery, Kerman University of Medical Sciences, Kerman, Iran 4Department of pathology, Medical school, P.O Box 444 Kerman University of Medical Sciences, Kerman, Iran
Correspondence Author E mail : m.s.sadjad@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320331
Article Received on : March 22, 2016
Article Accepted on : April 30, 2016
Article Published : 27 May 2016
In this study, biocompatibility of nano composites of silica doped in HA/collagen was compared against those doped in HA/gelatin. For this purpose, hydrothermal samples were synthesized before being investigated by X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR). Size and morphology of the samples were further characterized by scanning and transmission electron microscopy (SEM). Moreover, energy-dispersive X-ray spectroscopy (EDS) was used to undertake an elemental analysis on the prepared composites. FTIR spectras confirmed chemical structure of the nano composites and EDS patterns showed that, even though the nano composites of silica doped in HA/collagen had equivalent Ca/P ratios to the theoretical value as repeated for HA chemical structure of Ca10 (PO4)6(OH) 2, corresponding Ca/P ratios to the nano composites of silica doped in HA/gelatin were just close to the theoretical value. A comparison on the obtained XRD patterns showed that, the nano composites of silica doped in HA/collagen had their crystallite size smaller than that of the nano composites of silica doped in HA/gelatin. FESEM images indicated smaller particle sizes for the nano composites of silica doped in HA/collagen, as compared to those doped in HA/gelatin. Reduced crystallite size and decreased particle size are known to be associated with larger contact surface in chemical reactions, leading to better pharmacological efficacy in terms of bone repair.
KEYWORDS:hydroxyapatite; gelatin; collagen; silica; nanocomposite
Download this article as:| Copy the following to cite this article: Najafizadeha F, Sadjadia M. A. S, Fatemib S. J, Mobarakehc M. K, Afshard M. R. A Comparison Between Biocompatibilities of Nanocomposites of Silica Doped in HA/Collagen and Those Doped in HA/Gelatin. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Najafizadeha F, Sadjadia M. A. S, Fatemib S. J, Mobarakehc M. K, Afshard M. R. A Comparison Between Biocompatibilities of Nanocomposites of Silica Doped in HA/Collagen and Those Doped in HA/Gelatin. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=16813 |
Introduction
Improving biomaterials for enhanced osseointegration and tissue engineering (for bone remodeling) represents an international research target. Some of the most significant approaches to such a target have been reviewed in a special issue of Science1. During the last decade, a considerable deal of attention has been directed towards the use of bioactive materials, where bioactivity is defined as interfacial bonding of an implant or a bioactive scaffold to tissue by forming a biologically active hydroxyapatite (HA) layer on the bioactive material surface 2. Among all calcium phosphate bioceramics, HA (with the chemical formula of Ca10 (PO4)6(OH) 2) is the most extensively used, biocompatible ceramic material for bone tissue engineering, as it has its chemical composition well-similar to those of the bone mineral phase 3. A recently established materials concept of biomimetic composites based on silica, collagen, and calcium phosphates was adapted for the preparation of porous scaffolds suitable for tissue engineering applications 4-6. Synthetic biodegradable scaffolds are becoming highly promising materials for bone substitution and regeneration 7. They can provide an adequate structural support and a 3-D system on which cells can grow and migrate while the tissue regenerates, acting as a temporary extracellular matrix inducing the natural process of tissue regeneration and development 8. The use of bioactive HA coatings on metal implant surfaces have been reported to improve fixation in orthopedic implants, especially during early stages of healing 9, 10, 11. The requirement for biomaterials to assist in many medical applications replacing original musculoskeletal tissues and improve quality of life is rapidly increasing. HA has been widely used, as a bone graft material, in a range of medical and dental applications. However, pure HA suffers from low reactivity with existing bone 12, 13. HA and calcium-deficient HA in nano-particulate form are beginning to play significant roles in bone-related therapies owing to their unique functional properties including high surface area-to-volume ratio and ultrafine structure, which are similar to those of biological apatite. This similarity is of importance in cell–biomaterial interactions 14-19.
Experimental
Synthesis of nanocomposites of silica doped in HA/collagen
In situ synthesis of HA rods was carried out in a collagen matrix. In order to prepare modified HA, 2.8 g of collagen was mixed with 70 ml of distilled water while continuously applying a magnetic stirrer at its maximum speed at around 70°C. After stirring for 2 h , 80 ml of 0.1 M calcium chloride solution was gradually added to the prepared biopolymer solution. The solution was subsequently stirred for 1 h before introducing 48 ml of 0.1 M sodium phosphate in a drop wise fashion while stirred at a pH value of 10. The solution was placed in an oven at 100°C and kept for half an hour before filtering the formed precipitate. Once finished with preparing collagen-based HA nanocomposite, 30 cc of double-distilled water along with 7.0 g of Na2SiO3 were mixed in a beaker and then introduced into the solution while stirred for half an hour at 36.5°C. The samples were incubated at 36.5°C for 72 h after which time they were filtered and washed with double-distilled water, and then were dried at room temperature.
Synthesis of nanocomposites of silica doped in HA/gelatin
All synthesis steps explained in Section 2.1 were repeated with gelatin instead of collagen.
Results and Discussion
Fourier transform infrared spectroscopy (FT-IR)
Fig. 1 provides a comparison between FT-IR spectrum captured for synthesized nanocomposites of silica doped in HA/collagen (Fig. 1 A) and that of HA/gelatin (Fig. 1 B).
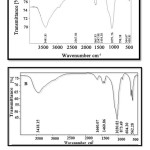 |
Figure 1: FT-IR spectra for nanocomposites of silica doped in A) HA/collagen, and B) HA/gelatin. |
The peaks at of 562 cm-1 at 603 cm-1 specify crystal structure of HA, while the peak at 1617 cm-1 specifies N-H, C- H stretching in collagen. The peak within 3200-3500 cm-1 attributes to the absorption of H2O on HA, with the one at 1010 cm-1 representing P-O stretching. The peak at
875 cm-1 specifies C-O stretching related to CO2 content of the air. The peak at 1175 cm-1 (C-H, N-H) refers to stretching amine (II), confirming the presence of HA/collagen. Non-presence of peaks related to the vibration absorption of Si-H (2100 cm-1) and Si-O bonds (1100 cm-1) in the spectrums proves the silica sample to be doped.
Table 1 reports several important infrared peaks assigned to the synthesized nanocomposites, confirming the formation of both products.
Table 1: Important Infrared Peaks Assigned To The Synthesized Nanocomposites.
|
Nanocomposites |
The wavelength cm-1 |
|
Crystal structure of HA |
464, 605, 566 |
|
P-O Stretching |
1036 |
|
O-H Stretching |
3851 |
|
N-H stretching |
3443 |
|
C=O vibration |
1651 |
Field emission scanning electron microscopy (FESEM)
Fig. 2 comes with FESEM spectra for the synthesized silica nanocomposites doped in HA/gelatin (A) and HA/collagen (B). Table 2 indicates particle sizes for synthesized silica nanoparticles doped in HA/gelatin (54.07 nm) and HA/collagen (46.85 nm).
Table 2 : A Comparison Between Particle Sizes of The Two Nanocomposite Samples.
|
Sample |
Particle size |
|
HA +Si+ gelatin |
54.07 nm |
|
HA +Si+ collagen |
46.85 nm |
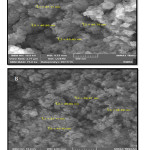 |
Figure 2: FESEM images of silica nanoparticles doped in A) HA/gelatin, and B) HA/collagen.
|
X-ray diffraction (XRD)
Fig. 3 shows XRD patterns for silica nanoparticles doped in HA/collagen (A) and HA/gelatin (B). The XRD peaks confirm crystalline planes of HA crystal system 20. On the other hand, the presence of silica dope in nanocomposites cannot change the HA crystal system
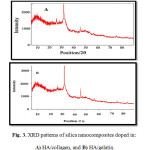 |
Figure 3: XRD patterns of silica nanocomposites doped in A) HA/collagen, and B) HA/gelatin. |
Table 3 shows crystallite sizes of the synthesized silica nanoparticles doped in HA/gelatin (10.73 nm) and HA/collagen (4.19 nm).
Table 3 : A Comparison Between Crystallite Sizes Of The Nanocomposite Samples.
|
Samples |
Crystallite size [Å] |
Crystallite size [nm] |
|
(HA+ Gelatin +Si) |
1073 |
10.73nm |
|
(HA+ Collagen +Si) |
419 |
4.19nm |
Energy-dispersive X-ray spectroscopy (EDS)
Elemental analysis were undertaken via energy-dispersive X-ray spectroscopy (EDS), so as to confirm nanocomposite preparation. Fig. 4 and Table 4 present the results obtained for the synthesized silica nanocomposites doped in HA/gelatin, while Fig. 5 and Table 5 come with the corresponding results to silica nanoparticles doped in HA/collagen. The presence of silica in Figs. 4 and 5 and Tables 4 and 5 confirms the doping of silica in synthesized nanocomposites. Continuing with the investigations, Ca/P ratios were calculated using the amounts of calcium and phosphorus. Accordingly, Ca/P ratios of 1.59 (close to theoretical value) and 1.67 (equivalent to the theoretical value) were obtained for silica nanoparticles doped in HA/gelatin and in HA/collagen, respectively.
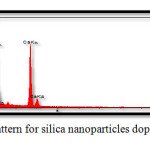 |
Figure 4: EDS pattern for silica nanoparticles doped in HA/gelatin Click here to View figure |
Table 4 :EDS Quantitative Results For Silica Nanoparticles Doped In HA/Gelatin.
| Elt | W% | A% |
| C | 12.11 | 18.82 |
| O | 49.38 | 57.62 |
| Na | 7.69 | 6.69 |
| Si | 8.36 | 5.55 |
| P | 8.67 | 4.90 |
| Ca | 13.79 | 6.42 |
| 100.0 | 100.0 |
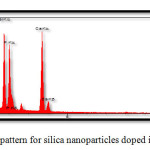 |
Figure 5: EDS pattern for silica nanoparticles doped inHA/collagen. |
Table 5:EDS Quantitative Results For Silica Nanoparticles Doped In HA/Collagen.
| Elt | W% | A% |
| C | 10.36 | 16.68 |
| O | 48.24 | 58.27 |
| Na | 3.67 | 4.79 |
| Si | 8.70 | 5.99 |
| P | 10.85 | 5.54 |
| Ca | 18.13 | 8.74 |
| 100.0 | 100.0 |
Table 6: A Comparison Between Ca/P Ratios Of The Two Nanocomposite Samples.
|
Samples |
Ca/P ratio |
Comments |
|
HA + Si + gelatin |
1.59 |
Close to the theoretical value |
|
HA + Si + collagen |
1.67 |
Equivalent to the theoretical value |
Conclusion
In this study, two hydrothermal HA nanocomposites were synthesized with their biocompatibilities investigated. According to the FTIR results, the synthesized HA nanocomposites had their chemical structures confirmed. Furthermore, based on the results of EDS method in terms of calcium-to-phosphorus ratios, the nanocomposites of silica doped in HA/collagen were observed to exhibit a Ca/P ratio of equivalent to the theoretical value as repeated for HA chemical structure of Ca10 (PO4)6(OH)2. Moreover, the results of spectroscopic XRD and SEM showed that the silica nanocomposites doped in HA/collagen were composed of smaller crystallite and particles than those of the silica nanocomposites doped in HA/gelatin. As reducing crystallite size and particle size increase contact surface, the silica nanocomposites doped in HA/collagen are expected to exhibit higher levels of reactivity in chemical reactions, making them of better pharmacological efficacy when it comes to bone repair.
References
- Sadjadia M.S., Ebrahimia H.R., Meskinfamb M., Zarec K., , Materials Chemistry and Physics., 2011 130, 67– 71.
CrossRef - Bizari D., Rabiee M., Moztarzadeh F., Tahriri M., Alavi S.H., Masaeli R., Bizari D., Rabiee M., Moztarzadeh F., Tahriri M., Alavi S.H., Masaeli R., Ceramics – Silikáty, 2013, 57 (3) 201-209.
- Farzadia A., Bakhshib F., MehranSolati N., MitraAsadi-Eydivanda, Noor AzuanabuOsmana, Ceramics International, 2014 (40)6021–6029.
- Heinemann S. , Heinemann C. , Jäger M. , Neunzehn J. , Wiesmann H.P. , Hanke T. , ACS Appl. Mater. Interfaces, 2011, 3 (11), 4323–4331.
CrossRef - Vallet-Regi M. and Arcos D., Materials Chemistry, 2005,. 15, pp. 1509-1516.
CrossRef - Cazalbou S., Combes C. and Rey C., Key Engineering Materials, 2001,. 92, . 13, . 192-195.
- Arcos D, Boccaccini AR, Bohner M, Díez-Pérez A, Epple M, Gómez-Barrena Acta Biomater, 2014, 10:1793–805.
CrossRef - Martínez-Vázquez F.J., Cabañas M.V. a, Paris J.L., Lozano D., Vallet-Regí M., Acta Biomaterialia 2015, (15) 200–209.
CrossRef - Degroot K., Geesink R., Klein C. and Serekain P., Journal of Bio- medical Materials Research, 1992,. 21,. 12. 1375-1381.
- Jaffe W. and Scott D.,” Journal of Bone and Joint Surgery, 1996, 78,. 12,. 1918-1934.
CrossRef - Mirjam Lilja., Lindahl Carl, Wei Xia., Håkan Engqvist., Maria Strømme1., Journal of Biomaterials and Nanobiotechnology, 2013, 4, 237-241.
CrossRef - Ducheyne P., Radin S., King L., J Biomed Mater Res, 1993, 27:25–34.
CrossRef - Klaudia Paljar., Sebastijan Orlić., Emilija Tkalčec., Hrvoje Ivanković., Marulićev trg, 2008,19,. 177.
- Webster T.J., Siegel R.W., Bizios R., Biomaterials, 2000; 21:1803–10.
CrossRef - Elliot J.C., 1994, Amsterdam: Elsevier Science B.V;.
- Natalia Davidenko., Raúl G., Carrodeguas, Carlos Peniche., Yaimara Solís., Acta Biomaterialia 2010, (6) 466–476.
CrossRef - Kokubo T., Kim H.M., Kawashita M., Biomaterials, 2003, 24:2161–75.
CrossRef - Doi Y., Horiguchi T., J Biomed Mater Res, 1996, 31:43–9.
CrossRef - Rusu V.M., Ng C.H., Wilke M., Tiersch B., Fratzl P., Peter M.G., Biomaterials, 2005, 26(26):5414–26.
CrossRef - Adrian Paz., Dainelys Guadarrama., Mónica López., Jesús E., Quim. Nova, 2012, 35(9), 1724-1727.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









