Removal of Bisphenol A using Antimony Nanoparticle Multi-walled Carbon Nanotubes composite from aqueous solutions
Mohammad Taghi Samadi1, Reza Shokoohi1, Ali Poormohammadi2, Ghasem Azarian1, Motahare Harati3 and Samane Shanesaz1*
1Department of Environmental Health Engineering, Faculty of Health and Research Center for Health sciences, Hamadan University of Medical Sciences, Hamadan, Iran. 2Social Development and Health Promotion Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran. 3Department of Environmental Health Engineering, School of public Health, Iran University of Medical Science, Iran, Iran. Corresponding author email: shanesaz.samane@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320227
Article Received on :
Article Accepted on :
Article Published : 29 Apr 2016
This study focuses on preparing Antimony Nano particle Multi-walled Carbon (ANMWC) composite as an effective adsorbent and then the effect of produced composite in BPA removal from aqueous solutions was studied. ANMWC were prepared using chemical method and characterized with X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR) and Brunauer–Emmett–Teller (BET). Moreover, the removal efficiency of prepared AMWCNT and Nano particle Multi-walled Carbon (MWCNT) in removal of Bisphenol A was investigated. Results revealed that the BPA removal efficiency by AMWCNT increased from 80 to 93 % with the increase of contact time 5 to 60 min. The maximum removal efficiency for the both adsorbents was seen at pH 7, which was 85% for MWCNT and 95% for ANMWC composite. According to the results obtained, pHzpc for both adsorbents was7. Results showed that the adsorption process followed the pseudo-first order model with a high correlation value and BPA adsorption on MWCNT followed the Langmuir isotherm model.
KEYWORDS:Bisphenol; Adsorption; Nanoparticle Multi-walled Carbon Nanotubes; composite
Download this article as:| Copy the following to cite this article: Samadi M. T, Shokoohi R, Poormohammadi A, Azarian G, Harati M, Shanesaz S. Removal of Bisphenol A using Antimony Nanoparticle Multi-walled Carbon Nanotubes composite from aqueous solutions. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Samadi M. T, Shokoohi R, Poormohammadi A, Azarian G, Harati M, Shanesaz S. Removal of Bisphenol A using Antimony Nanoparticle Multi-walled Carbon Nanotubes composite from aqueous solutions. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15655 |
Introduction
Bisphenol A (BPA) is one the most widely used chemical compounds as a raw material in the product of polycarbonate and epoxy resins 1. BPA can release into the environment through its use and handling, and permitted discharges 2. Recently, this compound and its derivatives have been found to be widely distributed in the natural environment, as well as in surface water 3. Due to the high toxicity of phenolic compounds to humans, their removal has been taken a huge attention of many researchers 4.
In recent years, nanotechnology has been taken into huge consideration as a promising technology to treat water. According to the literature, Multi-walled carbon nanotubes (MWCNTs) have shown an ability to efficiently remove various organic pollutants such as dioxins, polychlorinated dibenzo-furans and biphenyls from aqueous environments5, 6.
Nowadays, considerable efforts have been made to produce metal-carbon nanocomposite materials, not only because the carbonic compound improves the mechanical properties of the composites, but also because the produced composites possess the properties of individual components with a synergistic effect 7, 8.
Therefore, the present work focuses on preparing ANMWC composite as an effective adsorbent and then the effect of produced composite in BPA removal from aqueous solutions was studied. ANMWC were prepared using chemical method and characterized with X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR) and Transmission Electron Microscope (TEM).
Materials and Methods
Chemicals
Bisphenol-A was obtained from Merck (Germany). The MWCNT used in this study was obtained from Nutrieno Co. (Tehran, Iran, http://parscenter.com/Company/CMP645327). Sodium dodecyl sulfate, Antimony powder, Sodium sulfate anhydrous, Sodium hydride powder, Sodium Borohydride powder and other chemicals were purchased from Merck.
Table 1: Characteristics of MWCNTs
|
Parameter |
Unit |
Amount |
|
Appearance |
– |
Black powder |
|
External diameter |
nm |
20-30 |
|
Length |
µm |
30 |
|
Purity carbon |
% |
95 |
|
specific surface area |
m2/g |
110 |
|
Density |
g/cm3 |
2.1 |
Preparation and Characterization of adsorbents
To produce Antimony Nanoparticle Multi-walled Carbon (ANMWC) composite Ultrasonic device was used. The device consist of a stainless steel reactor equipped with a transformer and an ultrasonic wave generator. Table 2 shows the characteristics of used Ultrasonic device.
To prepare ANMWC composite, 100 mg of nanoparticle multi-walled carbon and 40 mg of sodium dodecyl sulfate (as surfactant) were dissolved in 100 ml of pure ethanol. The solution was mixed by Ultrasonic device for 20 min. Next, 80 mg of Sodium Borohydride was added to the solution. Again, the mixtures were mixed for 20 min. Then Antimony solution (17.5 mmol/L) were gradually added to the nanoparticle multi-walled carbons. Finally, the products were placed into the Ultrasonic devise for 1 h under stirring conditions. The products were then filtered and washed with doubly distilled water and then dried at 60 ˚C in an oven for 12 h 9.
Table 2: Characteristics of used Ultrasonic device
|
model |
LUC-405 |
|
time |
0-99 min |
|
temperature |
0-50 0c |
|
Frequency |
40 KHZ |
|
capacity |
5 L |
|
volume capacity |
300×55×150 mm |
|
useful volume |
300×285×255 mm |
|
power |
350 |
|
chamber material |
Stainless steel |
|
devise body material |
ABS |
|
country |
Korea |
|
voltage |
100 to 240v-AC,50/60Hz |
In order to obtain the characterization of adsorbent X-Ray Diffraction (XRD, ITALSTRUCTRE, model ADP 2000, Italy) was used. Fourier Transform Infrared Spectroscopy (FTIR, Perkin Elmer Spectrum) was employed in order to determine the functional groups and the chemical structure on surface area of adsorbent materials before and after modification. Moreover, Transmission Electron Microscope (TEM) was used in order confirm the adsorbent modification. Moreover, pHzpc of the synthesized composite and nanoparticle multi-walled carbon was determined using the pH drift method 10.
Batch experiments
To determine the optimum conditions for BPA removal by ANMWC composite, the effect of some operational parameters on the adsorption efficiency was studied. Experimental stages are summarized in Table 3 10, 11, 12,13,14.
Table 3: Experimental set-up (experiment steps)
|
Parameters |
Unit |
Range |
|
|
1 |
Contact time |
min |
50, 80, 100, 150 and 200 |
|
2 |
pH |
– |
3, 7 and 10 |
|
3 |
Synthesized adsorbent concentration |
mg/L |
100, 200 and 400 |
|
4 |
Initial BPA concentration |
mg/L |
20, 60 and 100 |
BPA residual concentration
After adsorption process BPA residual concentration was measured using a spectrophotometer (Uv/Vis spectrometer-DR 5000, Germany) at a wavelength of 276 nm [15, 16]. Eventually, BPA efficiency was calculated according to the following equation [12].
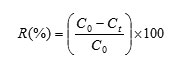
C0: initial concentration of the BPA (mg/L)
Ct: instant concentration of the BPA (mg/L)
R%: Percentage of BPA removal
Results and Discussion
The XRD patterns are shown in fig.1 (a, b). As can be seen in fig.1 (a), a peak around 25 indicated the presence of carbon, the peak around 43, indicated the presence of oxygen and peaks between 65 and 85 indicated the presence of antimony. Fig. 1 (b) and the presence of antimony element in the composition of MWCNTs confirms the claim that the ANMWC composite was properly prepared. This result is consistent with the finding of Fernando et al 9.
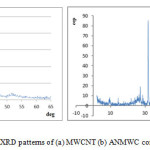 |
Figure 1: XRD patterns of (a) MWCNT (b) ANMWC composite
|
Characterization of adsorbents
Fig. 2 shows the FTIR spectra of both adsorbents (MWCNT and ANMWC composite) before and after adsorption process. As revealed, a broad peak around 3400 cm-1 correspond to the presence of O-H groups on the MWCNT surface. This figure showed peaks at 1623 and 1637 cm-1 which indicted the presence of aromatic structures C=O in the MWCNT. These results are consistent with the results of the FTIR spectrum obtained by Zazoli et al. on the application of L-cysteine functionalized single-walled carbon nanotubes for removing mercury from aqueous solutions 15. After adsorption process a peak around 1223 cm-1 indicated the presence of phenolic band C-O, which indicated that BPA has been adsorbed on the adsorbent surface. In spectrum of the carbon nanotubes modified with antimony, a peak at 2400 cm-1 indicated the presence of C-H stretching.
 |
Figure 2: FTIR spectra of MWCNT (a) before and (a) after adsorption process |
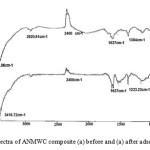 |
Figure 3: FTIR spectra of ANMWC composite (a) before and (a) after adsorption process |
Fig. 4 shows the TEM spectra of MWCNT and ANMWC composite. As revealed antimony particles have been located well on the MWCNT surface.
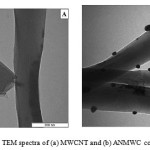 |
Figure 4: TEM spectra of (a) MWCNT and (b) ANMWC composite |
Contact time
Contact time is one of the most important parameters in adsorption process. Considering that the adsorption process is one of equilibrium reactions, so the contact time plays an important role in reaction progress. As the adsorbates can easily access to absorption sites, the process requires less contact time to reach its maximum absorption 18,19. On the other hand, as the adsorbates cannot easily access to the adsorption sites, required contact time increased. Fig. 5 shows the effect of contact time on adsorption efficiency. Results revealed that the BPA removal efficiency by MWCNT increased from 51 to 87 % with the increase of contact time 5 to 60 min. In the mentioned range of contact time, the ANMWC composite efficiency in BPA removal increased from 80 to 93 %. As can be seen, the efficiency of ANMWC composite was better in BPA removal than that of MWCNT alone. This can be related to the influence of Antimony coated on the MWCNT surface. Dehghani et al. suggested that BPA removal increased with increasing contact time, which is in line with our result 14. This result is also consistent with the finding of Samadi et al 20.
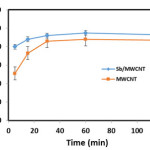 |
Figure 5 Click here to View figure |
Effect of solution pH
Due to the direct effect of solution pH on the adsorbent surfaces and the degree of ionization of pollutants, it considered as the most important parameter in adsorption process 21. Theoretically, pH can be affected on BPA absorption. As shown in fig.5, at acidic conditions, the removal efficiency of BPA increased gradually with increasing pH. The maximum removal efficiency was seen at pH 7, which was 85% for MWCNT and 95% for ANMWC composite. By contrast, with increasing pH values from 7 to 10, the removal efficiency for both adsorbents decreased obviously. This can be attributed to the structure and pHzpc of adsorbent. According to the results obtained, pHzpc for both adsorbents was7. When the pH of the solution is higher than the pHzpc, absorbent surface becomes negatively charged and can adsorb well cations by the electrostatic reactions. On the other hand, at pH values higher than the pHzpc, absorbent surface becomes positively charged and absorbs well anions. Considering the negative charge of BPA surface at alkaline conditions, the adsorption rate of BPA decreased. In contrast, because of positive charge of BPA at acidic conditions, the adsorption rate of BPA increased. In addition, at alkaline pH, the degradation of OH– groups of phenolic compounds such as BPA, prevents the formation of hydrogen bonds between adsorbed BPA molecules on the surface of the nanotubes and molecules dissolved in solution and as a result, adsorption efficiency decreased 22.
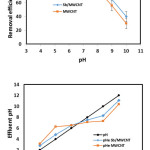 |
Figure 6 Click here to View figure |
Adsorption Isotherms and Kinetics Models
According to the results, the correlation coefficient (R2) for both the absorbents in pseudo-first order model was relatively low. So, the pseudo-first order model did not fit with the experimental data well. Results showed that the adsorption process followed the pseudo-first order model with a high correlation value. Pseudo-first order model is in accordance to the adsorption capacity and it is used when the adsorption occurs using diffusion mechanism through a boundary layer. The pseudo-second order model showed that the chemical adsorption mechanism is dominant and controller and acts as a moderator in the adsorption process 19.
In the present study, Langmuir and Freundlich isotherm models were investigated and the results of this stage are shown in Table 4. As can be seen here, in BPA adsorption on MWCNT, the correlation coefficient of Langmuir isotherm model (0.96) was higher than Freundlich isotherm model (0.82). Therefore, BPA adsorption on MWCNT followed the Langmuir isotherm model. Langmuir isotherm is the most common used isotherm to investigate the adsorption processes. This isotherm is based on the single-layer adsorption 23. This isotherm indicated that adsorbate molecules were adsorbed on the specific points of adsorbent surface, which are called absorption sites.
The energy of adsorbates in each of adsorption sites is same and this not attributed to the presence or absence of adjacent adsorbed molecules. This assumption suggested that the energy levels are quite the same. Adsorption process is topically and occurred by colliding the adsorbates with empty sites. Finally, desorption rate depends only on the amount of material adsorbed on the surface 19. a and b are Langmuir parameters, which indicated the maximum absorption capacity and correlation energy. While, the main feature of Langmuir isotherm is a dimensionless parameter called equilibrium (RL). The effectiveness of adsorption process in Langmuir model is determined by these parameters. 0<RL<1 indicate the suitable absorption, 1<RL indicate unsuitable adsorption, and RL=0 indicate irreversible absorption 24. According to the RL value (0.046), which was obtained from the calculations (Table 4), the absorption process of BPA on MWCNT was a suitable process.
According to the results, the correlation coefficient of Freundlich isotherm model (n=0.93) for the BPA absorption on ANMWC composite was higher than correlation coefficient of Langmuir isotherm model (0.81). As a result, the adsorption process followed the Freundlich isotherm model. Freundlich model defines the adsorption of adsorbates on heterogeneous surfaces and states that the adsorbates are adsorbed on the adsorbent as multi-layer. Kf and n are the constants, which are related to the adsorption capacity and absorption rate. The values of n<1 indicate unsuitable adsorption, 1<n<10 indicate a suitable adsorption, n>1 indicate stronger interactions between the absorbent and adsorbate, and n=1 indicates that the adsorption energy is the same for all sites (25). According to the n value (n=1.36), BPA absorption on ANMWC was suitable and there was a moderately strong interaction between adsorbate and adsorbent.
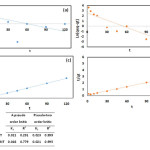 |
Figure 7 Click here to View figure |
Conclusion
The present focuses on preparing ANMWC composite as an adsorbent and then the effect of produced composite and MWCNT in BPA removal from aqueous solutions was studied. ANMWC were prepared using chemical method and characterized with X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR) and Transmission Electron Microscope (TEM). Results revealed that the BPA removal efficiency by ANMWC increased from 80 to 93 % with the increase of contact time 5 to 60 min. The maximum removal efficiency was seen at pH 7, which was 85% for MWCNT and 95% for ANMWC composite. According to the results obtained, pHzpc for both adsorbents was7. Results showed that the adsorption process followed the pseudo-first order model with a high correlation value and BPA adsorption on MWCNT followed the Langmuir isotherm model. According to the results, the both MWCNT and ANMWC composite are effective for removing pollutants from aqueous solutions. Moreover, the ANMWC composite can be used as a suitable and new adsorbent for removing organic pollutants from water environments.
Acknowledgment
Authors would like to thank the Hamadan Laboratory, Graduate School of Medical Sciences for financial support of this research. This article is extracted from the Master’s thesis (No, 9303131419).
References
- Kalmykova, Y.; Moona, N.; Strömvall, A.M.; Björklund K. Sorption and degradation of petroleum hydrocarbons, polycyclic aromatic hydrocarbons, alkylphenols, bisphenol A and phthalates in landfill leachate using sand, activated carbon and peat filters. J Water Res. 2014, 51, 246-257
CrossRef - Huang, Y.Q.; Wong, C.K.C.; Zheng, J.S.; Bouwman, H.; Barra, R.; Wahlström, B., et al. Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. J Environ Intern, 2012, 42, 91-99
CrossRef - Wang, J.; Schnute, W.C. Direct analysis of trace level bisphenol A, octylphenols and nonylphenol in bottled water and leached from bottles by ultra‐highperformance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom.2010, 24, 2605-10
CrossRef - Sui, Q.; Huang, J.; Liu, Y.; Chang, X.; Ji, G.; Deng, S. Rapid removal of bisphenol a on highly ordered mesoporous carbon. Environ Sci. 2011, 23, 177–82
CrossRef - Samadi, M. T.; Zolghadrnasab, H.; Godini, K.; Poormohammadi, A.; Ahmadian, M.; Shanesaz S. Kinetic and adsorption studies of reactive black 5 removal using multi -walled carbon nanotubes from aqueous solution. Der Pharma Chemica. 2015, 7, 267-274
- Heidari, Z.; Masrournia, M.; Sannavii Khoshnood, R. Fabrication a composite electrode based on MWCNT/Zeolite for potentiometric determination of Cr3+. Orient J Chem. 2016, 32, 627-635
CrossRef - Zhang, Y. J.; Yang, J.; Liu, B. L.; X. u, Y. D. Removal of Copper Ions from Water Using Chemical Modified Multi-walled Carbon Nanotubes. J Chem Soc Pakistan. 2014, 36, 841-847
- Mohammadkhani, Sh.; Gholami M. R.; Aghaie, M. Thermodynamic study of Cr+3 ions removal by “MnO2/MWCNT” nanocomposite. Orient J Chem.2016, 32, 592-599
CrossRef - Fernando, C.; Cesarinoa, I.; Cesarinoa, V.; Mascarob L, H.; Machadoa S, A. Carbon nanotubes modified with antimony nanoparticles: A novel material for electrochemical sensing. J Electrochim Acta ، 2012, 85, 560-565
CrossRef - Putra E, K.; Pranowo R.; Sunarso J.; Indraswati N.; Ismadji S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: mechanisms, isotherms kinetics. J Water Rese. 2009, 43, 2419-30
CrossRef - Saeedi, R.; Naddafi, K.; Nabizadeh, R. Lead (II) and cadmium (II) biosorption from aqueous by the Sargassum sp. biomass. J Sch Public Health Inst Public Health Res. 2007, 5, 13-24
- Kashitarash Isfahani, Z.; Samadi, M. T, Alavi, M.; Manuchehrpoor, N.; Bakhani, M. Efficiency of Carbon Nanotubes in Municipal Solid Waste Landfill Leachate (Case Study: Treatment of Hamadan Landfill Leachate). J .Water .Waste. 2011, 23, 67-72
- Samadi, M. T.; Kashitarash Esfahani, Z.; Ahangari, F.; Ahmadi, S. H.; Jafari, S. J. Nickel Removal from Aqueous Environments Using Carbon Nanotubes. J .Water .Waste. 2012, 24, 38-44
- Zazouli, M. A.; Balarak, D.; Mahdavi, Y.; Barafrashtehpour, M.; Ebrahimi, M. Adsorption of Bisphenol from Industrial Wastewater by Modified Red Mud. J health dev. 2013, 2, 1-11
- Zhijian, L. i.; Gondal, M. A.; Yamani, Z. H. Preparation of magnetic separable CoFe2O4/PAC composite and the adsorption of bisphenol A from aqueous solution. J. Saudi Chem. Soc. 2014, 18, 208-213
CrossRef - Dong, Y. I.; Deyi, W. U.; Chen, X.; Lin, Y. Adsorption of bisphenol A from water by surfactant-modified zeolite. J. Colloid Interface Sci. 2010, 348, 585-590
CrossRef - Zazouli, M. A.; Yousefi, Z.; Yazdani, J.; Tabarinia, H.; Tabarinia, F.; Akbari Adergani, B. Evaluation of L-Cysteine Functionalized Single-Walled Carbon Nanotubes On Mercury Removal from Aqueous Solutions. J Mazand Univ Med Sci. 2014, 24, 10-21
- Uddin, M.T.; Islam, M.S.; Abedin, M.Z. Adsorption of phenol from aqueous solution by water hyacinth ash. ARPN J. Eng. Appl. Sci. 2007, 2, 11-17
- Aksu, Z.; Yener, J. A comparative adsorption/biosorption study of monochlorinated phenols onto various sorbents. J Waste Manage. 2001, 21, 695-702
CrossRef - Asgari, G.; Seid Mohammadi, A. M.; Poormohammadi, Ahmadian, M. Removal of Cyanide from Aqueous Solution by Adsorption onto Bone Charcoal. Fresen Environ Bull. 2014, 23, 720-727
- Kakavandi, B.; Rezaei Kalantary, R.; Jonidi Jafari, A.; Esrafily, A.; Gholizadeh, A.; Azari, A. Efficiency of powder activated carbon magnetized by Fe3O4 nanoparticles for amoxicillin removal from aqueous solutions: Equilibrium and kinetic studies of adsorption process. Iran. J. Health & Environ. 2014, 7, 34-21
- Lin, D.; Xing, B. Adsorption of phenolic compounds by carbon nanotubes: role of aromaticity and substitution of hydroxyl groups. J. Environ. Sci. Technol. 2008, 42, 7254-59
CrossRef - Wu, J. H.; Yu, Q. Biosorption of 2, 4-dichlorophenol by immobilized white-rot fungus Phanerochaete chrysosporium from aqueous solutions. J Bioresour technol. 2007, 98, 253-259
CrossRef - Zheng, H.; Wang, Y.; Zheng, Y.; Zhang, H.; Liang, S.; Long, M. Equilibrium, kinetic and thermodynamic studies on the sorption of 4-hydroxyphenol on Cr-bentonite. J. Chem. Eng. 2008, 143, 117-23
CrossRef - Hameed, B.; Mahmoud, D.; Ahmad, A. Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (Cocos nucifera) bunch waste. J Hazard Mater. 2008, 158, 65-72
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









