An Efficient Approach for the Synthesis of Pyrazolo[1,2-a][1,2,4]Triazole-1,3-Diones Using an Electrochemical Cell
Khatereh Khandan-Barani1*, Mohammad Dodangeh2, Mehrnoosh Kangani2 and Malek-Taher Maghsoodlou2*
1Department of Chemistry, Islamic Azad University, Zahedan Branch, PO Box 98135-978, Zahedan, Iran
2Department of Chemistry, Faculty of Science, University of Sistan and Baluchistan, P. O. Box: 98135-674, Zahedan, Iran,
Corresponding author email: kh_khandan_barani@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320252
Article Received on :
Article Accepted on :
Article Published : 13 Apr 2016
A mild and efficient method was applied for the one-pot three-component synthesis of pyrazolo[1,2-a][1,2,4]triazole-1,3-diones from the condensation between arylaldehydes, malononitrile and 4-phenylurazole using electrolysis in an undivided cell in the presence of sodium bromide as an electrolyte. This procedure has many advantages such as : no need to catalyst, clean work-up, short reaction time and high yield. The products were obtained just with the simple filtration.
KEYWORDS:pyrazolo[1,2-a][1,2,4]triazole-1,3-dione; 4-phenylurazole; malononitrile; electrolysis
Download this article as:| Copy the following to cite this article: Khandan-Barani. K, Dodangeh M, Kangani M, Maghsoodlou M. T. An Efficient Approach for the Synthesis of Pyrazolo[1,2-A][1,2,4]Triazole-1,3-Diones Using an Electrochemical Cell. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Khandan-Barani. K, Dodangeh M, Kangani M, Maghsoodlou M. T. An Efficient Approach for the Synthesis of Pyrazolo[1,2-A][1,2,4]Triazole-1,3-Diones Using an Electrochemical Cell. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15305 |
Introduction
Many of heterocyclic compounds containing nitrogen have significant synthetic and biological importance, which act as polyfunctionalized fragments, agrochemicals, and pharmaceuticals [1–10]. Pyrazolourazoles as their fused derivatives with diverse structures have attracted much interest because of their wide range of biological properties such as analgesic, antibacterial, anti-inflammatory, antidiabetic, and psychoanaleptic activities [11–16] (Figure 1)., Many methods have been reported for their synthesis in recent years [17–26], and development of new approaches for their synthesis seems to be interesting challenge.
For preparing biologically active compounds in organic, combinatorial, and medicinal chemistry the electrosynthetically multicomponent reactions (EMCRs) have been used widely [27]. Due to the electron transfer between an electrode and the substrate molecules, the formation of highly reactive intermediates is achieved under mild conditions, avoiding reductive or oxidant agents as well as acids, bases and related waste by-products so that it can be one of the various fields in green chemistry [28]. In continue of our research on multi-component reactions [29-32],Herein, we report an efficient and green synthesis of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione derivatives using an electrochemical cell (scheme 1).
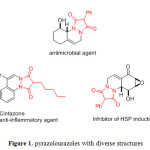 |
Figure 1: pyrazolourazoles with diverse structures |
![Scheme 1. Synthesis of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione derivatives with an electrochemical cell](http://www.orientjchem.org/wp-content/uploads/2016/04/Vol32_No2__An_KHAT_sch1-150x150.jpg) |
Scheme 1: Synthesis of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione derivatives with an electrochemical cell Click here to View scheme |
Materials and Method
All reagents and solvents were obtained from Fluka and Merck and used without further purification. TLC was performed on Silica–gel Polygram SILG/UV 254 plates. Melting points and IR spectra were measured on an Electro thermal 9100 apparatus and a JASCO FT-IR-460 plus spectrometer. Controlled-current coulometer and preparative electrolysis were performed using a SAMA potentiostate/galvanostate (Zahedan, Iran), respectively. The 1H NMR spectra were obtained on Bruker DRX-400 Advance instruments with DMSO. General procedure for the synthesis of 7-amino-1,3-dioxo-2-phenyl-2,3-dihydro-1H,5H-pyrazolo(1,2-a)(1,2,4)triazole-6-carbonitrile
A mixture of arylaldehyde (1 mmol), malononitrile (1mmol), 4-phenylurazole (1mmol ) and NaBr (0.05 g, 0.5 mmol ) in EtOH (20 mL) was stirred with a magnetic stirrer and electrolyzed in an undivided cell equipped with a graphite anode, and an iron cathode at ambient temperature under a constant current density of 10mA/cm2 ( electrodes square 5 cm2), until the catalytic quantity of 0.1 F/mol of electricity was passed. After electrolysis process, the mixture was filtered, then it was rinsed twice with cold ethanol to obtained corresponding product.
Analytical data for the selected compounds 7-amino-5-(3-nitrophenyl)-1,3-dioxo-2-phenyl-2,3-dihydro-1H,5H-pyrazolo(1,2-a)(1,2,4)triazole-6-carbonitrile (4b)
White powder, m.p: (>300˚C).; IR (KBr) (νmax/cm-1): 3430, 3314, 2189, 1766, 1715. 1H NMR (400 MHz, DMSO-d6) δH 6.094 (1H, s, CH), 7.47-8.37 (11H, m, H-Ar and NH2).
7-amino-5-(3-chlorophenyl)-1,3-dioxo-2-phenyl-2,3-dihydro-1H,5H-pyrazolo(1,2-a)(1,2,4)triazole-6-carbonitrile (4c)
White powder, m.p:(>300˚C).; IR (KBr) (νmax/cm-1): 3450, 3338, 2200, 1778, 1715.1H NMR (400 MHz, DMSO-d6) δH 6.09 (1H, s, CH), 7.47-8.37 (11H, m, H-Ar and NH2)
7-amino-5-(2-chlorophenyl)-1,3-dioxo-2-phenyl-2,3-dihydro-1H,5H-pyrazolo(1,2-a)(1,2,4)triazole-6-carbonitrile (4l)
White powder, m.p:(>300˚C).; IR (KBr) (νmax/cm-1): 3400, 3319, 1778, 1725. 1H NMR (400 MHz, DMSO-d6) δH 6.27 (1H, s, CH), 7.44-7.69 (11H, m, H-Ar and NH2).
Results and Discussion
To optimized the reaction conditions, the condensation between benzaldehyde, malononitrile and 4-phenylurazole was chosen as model reaction. The reactive mixture was stirred at room temperature, and this progress was monitored by TLC. The reaction is performed in alcoholic solvents in the presence of sodium bromide as an electrolyte. Various current quantities were applied under the mentioned conditions. As can be seen in Table 1, excellent conversions of the starting materials were obtained under 10 mA/cm2 current densities after 0.1 F/mol of electricity had passed. The current density of 10 mA/cm2, I = 50 mA, electrode surface Scheme 1.
Table 1: Optimization Of Reaction Conditions For The Synthesis Of 7-Amino-1,2,3,5-Tetrahydro-1,3-Dioxo-2,5-Diphenylpyrazolo[1,2-A][1,2,4]Triazole-6-Carbonitrile
|
Entry |
I (mA) |
Current density (mA/cm2) |
Time (min) |
catalyst |
Electricity passed (F/mol) |
Yields (%) |
|
1 |
5 |
1 |
250 |
– |
0.1 |
50 |
|
2 |
10 |
2 |
160 |
– |
0.1 |
65 |
|
3 |
20 |
4 |
50 |
– |
0.1 |
80 |
|
4 |
50 |
10 |
33 |
– |
0.1 |
93 |
|
5 |
75 |
15 |
23 |
– |
0.1 |
80 |
Using mentioned optimized reaction, the reaction were explored for the synthesis of a wide variety of pyrazolo[1,2-a][1,2,4]triazole-1,3-diones using aromatic aldehydes, malononitriles and 4-phenylurazol. The results are summarized in Table 2. As shown in Table 2, the products were obtained in excellent yields.
![Table 2. Synthesis Of Pyrazolo[1,2-A][1,2,4]Triazole-1,3-Dione Derivatives](http://www.orientjchem.org/wp-content/uploads/2016/04/Vol32_No2__An_KHAT_T2-150x150.jpg) |
Table 2: Synthesis Of Pyrazolo[1,2-A][1,2,4]Triazole-1,3-Dione Derivatives Click here to View table |
We proposed mechanism for the preparation of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione derivatives. First, deprotonation of an alcohol at the cathode leads to the formation of the alkoxide anion [33]. It’s subsequent reaction in solution with malononitrile gives rise to the malononitrile anion. Then, Knoevenagel condensation of aldehyde 1, with the malononitrile anion takes place in the solution with the elimination of water and the formation of the corresponding a-cyanocinnamo-nitrile derivatives A. The subsequent hydroxide-promoted Michael addition of 4-phenylurazole 3 to the electron-deficient Knoevenagel adduct A followed by intramolecular cyclization results in the corresponding products 4, with regeneration of the alkoxide anion as the last step, which continues the catalytic chain process by the interaction with the next molecule of malononitrile.
![Scheme 2 . Proposed mechanism for the synthesis of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione derivatives using electrolysis in the presence of sodium bromide as an electrolyte](http://www.orientjchem.org/wp-content/uploads/2016/04/Vol32_No2__An_KHAT_sch2-150x150.jpg) |
Scheme 2: Proposed mechanism for the synthesis of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione derivatives using electrolysis in the presence of sodium bromide as an electrolyte Click here to View scheme |
Conclusion
The pyrazolo[1,2-a][1,2,4]triazole-1,3-dione derivatives were synthesised in the presence of sodium bromide as an electrolyte under neutral and mild conditions. The main advantages of this method are the very short reaction time, high yields, simple work-up, use of non- hazardous organic solvent and catalyst.
Acknowledgment
We gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan.
References
- Franklin, E. C. Heterocyclic Chem Rev. 1935, 16, 305-361.
CrossRef - Bergstrom,F. W. Chem Rev 1944, 35, 77-277.
CrossRef - Lichtenthaler, F. W. Acc Chem Res. 2002, 35, 728-737.
CrossRef - Dömling, A.; Ugi, I.; Angew Chem Int Ed. 2000,39, 3168-3210.
CrossRef - Dömling, A. Chem Rev. 2006,106, 17-89.
CrossRef - Sheibani,H.; Babaie, M. Synth Commun. 2010, 40, 257.
CrossRef - Sheibani, H.; Seifi, M.; Bazgir,A. Synth Commun. 2009,39,1055-1064.
CrossRef - Guo,S.; Wang,S.; Li, J. Synth Commun. 2007, 37, 2111-2120.
CrossRef - Azarifar,D.; Maleki,B. Synth Commun. 2005, 35, 2581-2585.
CrossRef - Wang, S.; Ren, Z.; Cao, W.; Tong, W.; Synth Commun. 2001, 31, 673-677.
CrossRef - Bebernitz, G. R.; Argentieri, G.; Battle, B.; Brennan,C.; Balkan, B.; Burkey,B. F.; Eckhardt, M.; .Gao, J.; Kapa, P.; Strohschein,R. J.; Schuster,H. F.; Wilson, M.; Xu, D. D. J Med Chem. 2001, 44, 2601-2611.
CrossRef - Bekhit, A. A.; Fahmy H. T. Y.; Rostom, S. A. F.; Baraka, A.M. Eur J Med Chem. 2003, 38, 27-36.
CrossRef - Eid, A. I.; Kira, M. A.; Fahmy,H. H. J Pharm Belg. 1978, 33, 303-311.
- Park, H.A.; Lee, K.; Park, S. J.; Ahn, B.; Lee, J.C.; Cho, H. Y.; Lee, K. I. Bioorg Med Chem Lett . 2005, 15, 3307-3312.
CrossRef - Parmar, S. S.; Pandey,B. R.; Dwivedic,C.; Harbison, R. D. J Pharm Sci. 1974, 63, 1152-1155.
CrossRef - Takabatake, E.; Kodama, R.; Tanaka,Y.; Dohmori, R.; Tachizawa,H.; Naito, T. Chem Pharm Bull. 1970, 18, 1900.
CrossRef - Boldi,A. M.; Johnson, C. R.; Eissa, H. O. Tetrahedron Lett. 1999, 40, 619.
CrossRef - Arroya,Y.; Rodriguez, J. F.; Santos, M.; Sanz M.; Tejedor, A.; Vaco, I.; GarciaRuano,J. L. Tetrahedron: Asymmetry. 2004, 15, 1059-1067.
- Tanaka,S.; Seguchi, K.; Itoh, K. J Chem Soc, PerkinTrans. 1994, 1, 2335-2339.
CrossRef - Deghati,P. Y. F.; Wanner,M. J.; Koomen, G. J. TetrahedronLett. 1998, 39, 4561-4564.
CrossRef - Meehan, S.; Little, R. D. A. J Org Chem. 1997, 62, 3779-3781.
CrossRef - Menard,C.; Doris, E.; Mioskowski, C. Tetrahedron Lett. 2003, 44, 6591.
CrossRef - Nabid, M. R.; Tabatabaei Rezaei, S.J.; Ghahremanzadeh, R.; Bazgir, A.; Ultrason Sonochem. 2010,17, 159-161.
CrossRef - Azarifar, D.; Nejat-Yami, R.; Zolfigol, M. A. J. Hetero. Chem. 2013, 50, 1386-1390.
CrossRef - Adib, M.; Sayahi, M. H.; Mahmoodi, N.; Bijanzadeh, H. R.; Helv Chim Acta. 2006, 89, 1176-1180.
CrossRef - Ghahremanzadeh,R.; Shakibaei Imani, G.; Bazgir, A. Synlett. 2008,1129-1132.
- Elinson, M.N.; Merkulova, V.M.; Ilovaiskya, A.I.; Barba, F.; Batanero, B. Electrochim. Acta. 2011, 56, 8219-8223.
CrossRef - Elinson, M M.N.; Dorofeev, A.S. S.; Feducovich, K.; Nasybullin, R.F.; Gorbunov,S.V.; Nikishin, G.I. Electrochem. Commun. 2006, 1567-1571 (b) Elinson, M.N.; Dorofeev, A.S.; Nasybullin,R.F.; Nikishin,G.I. Synthesis. 2008, 1933-1937.
CrossRef - Mousavi, M.R; Hazeri, N.; Maghsoodlou, M. T.; Salahi, S.; Habibi-Khorassani, S. M. Chin. Chem. Lett. 2013, 24, 411 -414.
CrossRef - Khandan-Barani, K.; Maghsoodlou, M. T.; Habibi-Khorassani, S. M.; Hazeri, N.; Sajadikhah, S. S. J Chem Res, 2011, 231-238.
CrossRef - Khandan-Barani, K.; Maghsoodlou, M. T.; Habibi-Khorassani, S. M.; Hazeri, N.; Sajadikhah, S. S. Arkivoc, 2011, (xi), 22-28.
- Khandan-Barani, K.; Maghsoodlou, M. T.; Hazeri, N.; Habibi-Khorassani, S. M. Iran. J. Org. Chem, 2012, 4, 901-904.
- Elinson, M.N.; Ilovaisky,A.I.; Dorofeev, A.S.; Merkulova,V.M.; Stepanov,N.O.; Miloserdov, F.M.; Ogibin, Y.N.; Nikishin, G.I. Tetrahedron. 2007, 63, 10543-10548.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









