A simple and efficient route for synthesis of 2-alkylbenzothiazoles
Wijitra Waengdongbung, Viwat Hahnvajanawong* and Parinya Theramongkol
Natural Products Research Unit, Department of Chemistry and Center for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand. Corresponding author Email : viwhah@kku.ac.th
DOI : http://dx.doi.org/10.13005/ojc/320220
Article Received on :
Article Accepted on :
Article Published : 15 Apr 2016
The condensation of 2-aminothiophenol with aliphatic aldehydes in the presence of 4Å molecular sieves, followed by oxidation with silica gel supported pyridinium chlorochromate offers a simple and efficient route to 2-alkylbenzothiazoles.
KEYWORDS:2;3-dihydro-2-alkylbenzo[d]thiazoles; 2-alkylbenzothiazoles; silica gel supported PCC
Download this article as:| Copy the following to cite this article: Waengdongbung W, Hahnvajanawong V, Theramongkol P. A simple and efficient route for synthesis of 2-alkylbenzothiazoles. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Waengdongbung W, Hahnvajanawong V, Theramongkol P. A simple and efficient route for synthesis of 2-alkylbenzothiazoles. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15361 |
Introduction
Development of a straight forward method for synthesizing benzothiazoles and their derivatives is desired, since compounds containing benzothiazole moiety exhibit an array of biological activities, including, anticancer,1 antimicrobial,2 antituberculosis,3 antihypertensive,4 antiulcer,5 and also can be used as chemiluminescent agents6 and photosensitizers.7 Treatment of 2-aminothiophenol with aldehydes in the presence of catalysts such as RuCl3,8 Pt/Al2O3,9 CdS nanosphere (CdSNS),10 cetyltrimethyl ammonium bromide (CTAB),11 3,6-di(pyridine-2-yl)-1,2,4,5-tetrazine (pytz),12 bakers’ yeast13 and glucose oxidase (GOX)-chloroperoxidase (CPO)14 have been reported to give various 2-arylbenzothiazoles and some 2-alkylbenzothiazoles in satisfactory yields. In searching for an efficient catalyst-free synthesis of 2-substituted benzothiazoles, it was found that condensation of aromatic aldehydes with 2-aminothiophenol under melt reaction conditions afforded good to excellent yields of 2-arylbenzothiazoles under solvent-free conditions with no need of a catalyst. The reaction of aliphatic aldehydes, on the other hand, gave 2,3-dihydro-2-alkylbenzo[d]thiazoles as the sole products.15
We have examined the reaction of aliphatic aldehydes with 2-aminothiophenol and report herein our simple and efficient route to 2-alkylbenzothiazoles.
Experimental
Solvents were purified according to standard methods prior to use, while all other chemicals used were commercially available and used as received. Melting point was measured on a Sanyo Gallenkamp melting point apparatus and compared with that of known sample. IR spectra were recorded on a Perkin Elmer Spectrum One FT-IR Spectrometer. 1H and 13C NMR spectra were recorded using a VARIAN MERCURY plus (400 MHz FT NMR).
General procedure for the preparation of 2-alkyl-2,3-dihydrobenzo[d]thiazoles
To a stirred solution of aliphatic aldehyde (7.5 mmol) in dichloromethane (7.5 ml) was added 4Å molecular sieves (5.0 g). 2-Aminothiophenol (1) (0.63 g, 5.0 mmol) was added dropwise to the mixture and stirred altogether at room temperature for 1.5 – 2 h. After completion of the reaction, the reaction mixture was filtered to remove the molecular sieves. The solvent was evaporated under reduced pressure. The residue was purified by column chromatography on silica gel with 10% ethyl acetate/hexane as eluant to give the 2-alkyl-2,3-dihydrobenzo[d]thiazole.
2-Propyl-2,3-dihydrobenzo[d]thiazole (3a).
Yellow liquid; FTIR (neat, ν, cm-1) vmax 3464, 3359, 3064, 2954, 2928, 1607, 1583, 1464, 1307, 1118, 736 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.09 (1H, d, J = 7.6 Hz, 4-H), 6.97 – 6.89 (1H, m, 6-H), 6.76 (1H, dt, J = 7.5 Hz, J = 0.9 Hz, 5-H), 6.64 (1H, d, J = 7.8 Hz, 7-H), 5.27 (1H, t, J = 6.5 Hz, 2-H), 4.12 (1H, brs, NH), 1.86 (2H,dt, J = 10.2 Hz, J = 6.9 Hz, 1′-H), 1.46 (2H, sex, J = 8.0 Hz, 2′-H), 0.98 (3H, t, J = 7.4 Hz, 3′-H); 13C NMR (CDCl3) δ 146.7, 125.1, 122.0, 120.7, 110.8, 68.7, 40.7, 19.4, 13.8.
2-Methyl-2,3-dihydrobenzo[d]thiazole (3b)
Yellow liquid; FTIR (neat, ν, cm-1) vmax 3359, 2957, 2928, 2869, 1607, 1583, 1464, 1377, 1370, 736 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.08 (1H, d, J = 7.6 Hz, 4-H), 6.91 (1H, t, J = 7.6 Hz, 6-H), 6.76 (1H, t, J = 7.5 Hz, 5-H), 6.65 (1H, d, J = 8.3 Hz, 7-H), 5.39 (1H, q, J = 6.0 Hz, 2-H), 3.97 (1H, brs, NH), 1.60 (3H, d, J = 12.4, 6.0 Hz, 1′-H); 13CNMR (CDCl3) d 146.4, 125.2, 122.0, 121.0, 111.1, 63.9, 63.8, 24.4.
2-Ethyl-2,3-dihydrobenzo[d]thiazole (3c)
Yellow liquid; FTIR (neat, ν, cm-1) vmax 3354, 3065, 2962, 1607, 1581, 1464, 1400, 1378, 1117, 736 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.08 (1H, d, J = 7.6 Hz, 4-H), 6.92 (1H, t, J = 7.6 Hz,6-H), 6.75 (1H, t, J = 7.5 Hz, 5-H), 6.63 (1H, t, J = 8.5 Hz, 7-H), 5.22 (1H, t, J = 6.3 Hz, 2-H), 4.13 (1H, brs, NH), 1.88 (2H, quint, J = 7.1 Hz, 1′-H), 1.02 (3H, t, J = 7.4 Hz, 2′-H); 13C NMR (CDCl3) δ 146.7, 127.2, 125.1, 121.9, 120.6, 110.6, 70.2, 31.7, 10.2.
2-Isopropyl-2,3-dihydrobenzo[d]thiazole (3d)
Yellow liquid; FTIR (neat, ν, cm-1) vmax 3465, 3367, 3063, 2962, 2926, 1608, 1508, 1387, 1307, 1255, 745 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.08 (1H, d, J = 7.5 Hz, 4-H), 6.92 (1H, t, J = 7.6 Hz, 6-H), 6.73 (1H, t, J = 7.5 Hz, 5-H), 6.61 (1H, d, J = 7.8 Hz, 7-H), 5.16 (1H, d, J = 6.2 Hz, 2-H), 4.20 (1H, brs, NH), 2.06 – 1.95 (1H, m, 1′-H), 1.03 (6H, d, J = 6.7 Hz, 2′ and 3′-H); 13C NMR (CDCl3) d 147.3, 127.1, 125.0, 121.6, 120.2, 109.9, 75.0, 35.8, 18.7,18.3.
2-Hexyl-2,3-dihydrobenzo[d]thiazole (3e)
Yellow liquid; FTIR (neat, ν, cm-1) vmax 3371, 3064, 2924, 2853, 1584, 1516, 1463, 1373, 756, 729 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.06 (1H, d, J = 7.6 Hz, 4-H), 6.90 (1H, t, J = 7.6 Hz, 6-H), 6.73 (1H, t, J = 7.5 Hz, 5-H), 6.64 (1H, d, J = 7.7 Hz,7-H), 5.26 (1H, t, J = 6.5 Hz, 2-H), 4.08 (1H, brs, NH), 1.94 – 1.80 (2H, m, 1′-H), 1.48 – 1.18 (8H, m, 2′, 3′, 4′, and 5′-H), 0.90 (3H, m, 6′-H); 13C NMR (CDCl3) d 146.6, 125.8, 125.1, 121.9, 120.8, 110.8, 77.4, 77.1, 76.7, 68.9, 38.6, 31.7, 28.9, 26.1, 22.5, 14.0.
2-Benzyl-2,3-dihydrobenzo[d]thiazole (3f)
Yellow liquid; FTIR (neat, ν, cm-1) nmax 3361, 3061, 3027, 2924, 1583, 1465, 1311, 1276, 755, 698 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.38 – 7.23 (6H, m, Ar-H), 7.09 (1H, d, J = 7.6 Hz, 4-H), 6.93 (1H, t, J = 7.6 Hz, 6-H), 6.76 (1H, t, J = 7.5 Hz, 5-H), 6.62 (1H, d, J = 7.7 Hz, 7-H), 5.41 (1H, dd, J = 7.7 Hz, J = 6.0 Hz, 2-H), 4.17 (1H, brs, NH), 3.18 (1H, dd, J = 13.4, J = 8.1 Hz, 1′-H), 3.09 (1H, dd, J = 13.4, J = 5.6 Hz, 1′-H); 13C NMR (CDCl3) d 145.9, 137.0, 129.3, 128.8, 127.0, 125.3, 122.2, 120.6, 110.4, 77.3, 77.0, 76.7, 68.9, 45.0.
2-Phenylethyl-2,3-dihydrobenzo[d]thiazole (3g)
Yellow liquid; FTIR (neat, ν, cm-1) vmax 3357, 3060, 3025, 2920, 1581, 1466, 1400, 1223, 735, 694 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.34 – 7.27 (2H, m, 2′ and 6′-H), 7.22 (3H, t, J = 6.8 Hz, 3′, 4′ and 5′-H), 7.09 (1H, d, J = 7.5 Hz, 4-H), 6.91 (1H, t, J = 7.6 Hz, 6-H), 6.76 (1H, t, J = 7.5 Hz, 5-H), 6.64 (1H, d, J = 7.7 Hz, 7-H), 5.32 – 5.20 (1H, m, 2-H), 2.84 – 2.68 (2H, m, 1′-H), 2.20 (2H, dd, J = 14.5, 7.4 Hz, 2′-H); 13C NMR (CDCl3) δ 146.44, 140.81, 129.03, 128.53, 128.43, 127.57, 126.14, 125.16, 121.99, 120.90, 110.99, 67.93, 40.19, 32.20.
Preparation of silica supported PCC
The solid supported PCC was prepared by a procedure reported in the literature.13 To a solution of PCC (23.5 g, 109 mmol) in acetone (109 ml) was added silica gel 70 – 230 mesh (109 g) and the mixture was stirred at room temperature for 3 h. After removal of the solvent under reduced pressure, the resulting solid was dried at 100 oC for 2 h.
General procedure for the preparation of 2-alkylbenzothiazoles
To a stirred suspension of PCC on silica gel (2.6 g, 2.2 mmol) in dichloromethane (10 ml) was added 2-alkyl-2,3-dihydrobenzothiazole (2.0 mmol) and the mixture was stirred at room temperature for 30 min. After completion of the reaction, the resulting mixture was filtered on a thin Celite pad. The filtrate was poured into water and extracted with ethyl acetate (3´20 ml). The organic layer was dried (anh.Na2SO4), filtered, concentrated, and purified by column chromatography on silica gel using 10% ethyl acetate/hexane to give the 2-alkylbenzothiazole.
2-Propylbenzothiazole (4a)
Yellow liquid; FTIR (neat, ν, cm-1) vmax 3062, 2962, 2930, 2871, 2361, 1739, 1592, 1517, 1455, 1434, 1378, 1310, 757 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.97 (1H, d, J = 8.1 Hz, 4-H), 7.83 (1H, d, J = 8.0 Hz, 7-H), 7.44 (1H,t, J= 8.0 Hz, 6-H), 7.34 (1H, t, J= 8.0 Hz, 5-H), 3.09 (2H, t, J = 7.6 Hz, 1′-H), 1.91 (2H, dd, J = 15.0 Hz, J = 7.5 Hz, 2′-H), 1.06 (3H, t, J = 7.4 Hz, 3′-H); 13C NMR (CDCl3) δ 172.1, 153.3, 135.2, 125.8, 124.6, 122.5, 121.4, 36.2, 23.1, 13.7.
2-Methylbenzothiazole (4b)
Yellow liquid; FTIR (neat, ν, cm-1) vmax 3027, 2957, 2923, 2851, 1526, 1433, 1309, 1241, 1174, 759 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.95 (1H, d, J = 8.1 Hz, 4-H), 7.81 (1H, d, J = 8.0 Hz, 7-H), 7.44 (1H, t, J = 7.7 Hz, 6-H), 7.34 (1H, t, J = 7.6 Hz, 5-H), 2.83 (3H, s, CH3); 13C NMR (CDCl3) δ 166.9, 153.4, 135.6, 125.9, 124.7, 122.4, 121.3, 20.1.
2-Ethylbenzothiazole (4c)
Yellow liquid; FTIR (neat, ν, cm-1) vmax 3061, 2972, 2933, 1519, 1433, 1376, 1310, 1122, 756, 726 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.95 (1H, d, J = 8.2 Hz,4-H), 7.77 (1H, d, J= 8.0 Hz, 7-H), 7.39 (1H, t, J= 8.0 Hz, 6-H), 7.28 (1H, t, J= 8.0 Hz, 5-H), 3.10 (2H, m, 1′-H), 1.45 – 1.40 (3H, m, 2′-H); 13C NMR (CDCl3) δ 173.4, 153.2, 135.0, 125.8, 124.5, 122.4, 121.4, 27.7, 13.7.
2-Isopropylbenzothiazole (4d)
Yellow liquid; FTIR (neat, ν, cm-1) vmax 3063, 2966, 2928, 2870, 1517, 1459, 1439, 1309, 1035, 1006, 759 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.98 (1H, d, J = 7.9 Hz, 4-H), 7.85 (1H, dd, J = 7.9 Hz, J = 0.5 Hz, 7-H), 7.44 (1H, t, J= 8.0 Hz, 6-H), 7.34 (1H, t, J= 8.0 Hz, 5-H), 3.43 (1H, hept, J = 6.9 Hz, 1′-H), 1.48 (6H, d, J = 6.9 Hz, 2′ and 3′-H); 13C NMR (CDCl3) δ 178.6, 153.1, 134.7, 125.8, 124.5, 122.6, 121.5, 34.1, 22.8.
2-Hexylbenzothiazole (4e)
Yellow liquid; FTIR (neat, ν, cm-1) vmax3064, 2926, 2857, 1518, 1459, 1435, 1249, 1086, 1062, 758, 728 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.97 (1H, d, J = 8.1 Hz, 4-H), 7.83 (1H, d, J = 8.0 Hz, 7-H), 7.44 (1H, t, J = 7.7 Hz, 6-H), 7.34 (1H, t, J = 7.6 Hz, 5-H), 3.11 (2H, t, J = 7.7 Hz, 1′-H), 1.95 – 1.78 (2H, m, 2′-H), 1.47 – 1.07 (6H, m, 3′, 4′, and 5′-H), 0.89 (3H, t, J = 6.7 Hz, 6′-H); 13C NMR (CDCl3) δ 172.4, 153.6, 135.1, 125.8, 124.6, 122.5, 121.4, 34.3, 31.5, 29.7, 28.8, 22.5, 14.0.
2-Benzylbenzothiazole (4f)
Yellow liquid; FTIR (neat, ν, cm-1) vmax 3061, 3029, 2992, 2852, 1646, 1514, 1490, 1452, 1432, 757, 701 cm-1; 1H NMR (CDCl3, 400 MHz) δ 8.00 (1H, d, J = 8.2 Hz, 4-H), 7.79 (1H, d, J = 7.9 Hz, 7-H), 7.45 (1H, t, J = 7.5 Hz, 6-H), 7.41 – 7.27 (6H, m, ArH and 5-H), 4.44 (2H, s, 1′-H); 13C NMR (CDCl3) δ 171.2, 153.2, 137.2, 131.3, 129.1, 128.8, 128.5, 127.3, 125.9, 124.8, 122.8, 121.5, 40.6.
2-Phenylethylbenzothiazole (4g)
Yellow solid; mp 60 – 62 oC (lit.16 59-60 oC); FTIR (KBr, ν, cm-1)vmax 3058, 3027, 2923, 2852, 1516, 1451, 1432, 1118, 752, 696 cm-1; 1H NMR (CDCl3, 400 MHz) δ 7.99 (1H, d, J = 8.1 Hz, 4-H), 7.84 (1H, d, J = 8.0 Hz, 7-H), 7.46 (1H, t, J = 7.7 Hz, 6-H), 7.40 – 7.16 (6H, m, ArH and 5-H), 3.47 – 3.34 (2H, m, 1′-H), 3.26 – 3.13 (2H, m, 2′-H); 13C NMR (CDCl3) δ 170.9, 153.2, 140.2, 135.1, 128.6, 128.4, 126.4, 125.9, 124.8, 122.6, 121.5, 36.0, 35.5.
Results and Discussion
We initially performed the reaction of 2-aminothiophenol (1) with butanal (2a) in the absence of solvent at room temperature for 24 h. As shown in Scheme 1, isolated products were 2-propyl-2,3-dihydrobenzo[d]thiazole (3a) and 2-propylbenzothiazole (4a) in 40 and 25% yields, respectively. Carrying out this reaction in CH2Cl2 in the presence of 4Å molecular sieves at room temperature for 2 h,17 on the other hand, provided 2-propyl-2,3-dihydrobenzo[d]thiazole (3a) in 96% yield as the sole product.
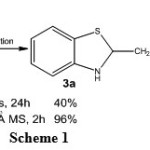 |
Scheme 1 Click here to View scheme |
Treatment of 2-aminothiophenol (1) with aliphatic aldehydes 2b-g in CH2Cl2 in the presence of 4Å molecular sieves at room temperature for 1.5-2 h also gave excellent yields of the corresponding 2-alkyl-2,3-dihydrobenzo[d]thiazoles 3b-g, as illustrated in Table 1.
![Table 1. Synthesis of 2-alkyl-2,3-dihydrobenzo[d]thiazoles 3b-g from the reaction of 2-aminothiophenol (1) with aliphatic aldehydes 2b-g in CH2Cl2 in the presence of 4Å molecular sieves](http://www.orientjchem.org/wp-content/uploads/2016/04/Vol32_No2_sim_WIJI_T1-150x150.jpg) |
Table 1: Synthesis of 2-alkyl-2,3-dihydrobenzo[d]thiazoles 3b-g from the reaction of 2-aminothiophenol (1) with aliphatic aldehydes 2b-g in CH2Cl2 in the presence of 4Å molecular sieves Click here to View table |
Since pyridinium chlorochromate (PCC) supported on silica gel have been successfully used as an oxidant for the oxidative cyclization of thiophenolic Shiff’s bases in CH2Cl2,18 we therefore treated 2-alkyl-2,3-dihydrobenzo[d]thiazoles 3a-g with silica gel supported PCC in CH2Cl2 at room temperature. As shown in Scheme 3, this treatment afforded excellent yields of the corresponding 2-alkylbenzothiazoles 4a-g in 20 min.
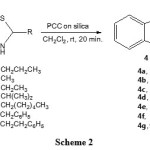 |
Scheme 2 Click here to View scheme |
Conclusion
The present two-step procedure involving condensation between 2-aminothiophenol and aliphatic aldehydes in the presence of 4Å molecular sieves followed by oxidation of the obtained 2-alkyl-2,3-dihydrobenzo[d]thiazoles using pyridinium chlorochromate (PCC) supported on silica gel as oxidizing agent provides a simple and efficient route to 2-alkylbenzothiazoles.
Acknowledgement
This work was financially supported by the Natural Products Research Unit, Department of Chemistry, Faculty of Science, Khon Kaen University, and the Center for Innovation in Chemistry (PERCH-CIC) and the Commission of Higher Education (CHE-RG), Ministry of Education.
References
- Easmon, J.; Pürstinger, G.; Thies, K.-S.; Heinisch, G.; Hofmann, G. J. Med. Chem. 2006, 49, 6343-6350.
CrossRef - Zajac, M.; Hrobárik, P.; Magdolen, P.; Foltínová, P.; Zahradník, P. Tetrahedron 2008, 64, 10605-10618.
CrossRef - Yamamoto, K.; Fujita, M.; Tabashi, K.; Kawashima, Y.; Kato, E.; Oya, M.; Iso, T.; Iwao, J. J. Med. Chem., 1988, 31, 919–930.
CrossRef - Seyhan, E.; Sultan, N.; Nilgun, A.; Noyanalpan, N. Arzneim. Forsch. 1997, 47, 410-412.
- Gungor, T.; Fouquet, A.; Teulon, J. M.; Provost, D.; Cazes, M.; Cloarec, A. J. Med. Chem.1992, 35, 4455–4463.
CrossRef - Yoshida, H.; Nakao, R.; Nohta, H.; Yamaguchi, M. Dyes Pigm. 2000, 47, 239-245.
CrossRef - Petkov, I.; Deligeorgiev, T.; Markov, P.; Evstatiev, M.; Fakirov, S. Polym. Degrad. Stab. 1991, 33, 53-66.
CrossRef - Fan, X.; Wang, Y.; He, Y.; Zhang, X.; Wang, J. Tetrahedron Lett. 2014, 51, 3493-3496.
CrossRef - Chaudhari, C.; Hakim Siddiki, S. M. A.; Shimizu, K. Tetrahedron Lett. 2015, 56, 4885-4888.
CrossRef - Das, S.; Samanta, S.; Maji, S. K.; Samanta, P. K.; Dutta, A. K.; Srivastava, D. N.; Adhikary, B.; Biswas, P. Tetrahedron Lett. 2013, 54, 1090-1096.
CrossRef - Yang, X. L.; Xu, C. M.; Chen, J. X.; Ding, J. C.; Wu, H. Y.; Su, W. K. J. Braz. Med. Chem. 2010, 21, 37-42.
CrossRef - Samanta, S.; Das, S.; Biswas, P. J. Org. Chem. 2013, 78, 11184-11193.
CrossRef - Pretap, U. R.; Mali, J. R.; Jawale, D. V.; Mane, R. A. Tetrahedron Lett. 2009, 50, 1352-1354.
CrossRef - Kumar, A.; Sharma, S.; Maurya, R. A. Tetrahedron Lett. 2010, 51, 6224-6226.
CrossRef - Sedaghat, N.; Naimi-Jamal, M. R.; Mokhtari, J. Curr. Chem. Lett. 2014, 3, 57-62.
CrossRef - Giorgioni, G.; Accorroni, B.; Di Stefano, A.; Marucci, G.; Siniscalchi, A.; Claudi, F. Med. Chem. Res. 2005, 14, 57-73.
CrossRef - Joly, G. D.; Jacobsen, E. N. J. Am. Chem. Soc. 2004, 126, 4102-4103.
CrossRef - Praveen, C.; Kumar, K. H.; Muralidharan, D.; Perumal, P. T. Tetrahedron 2008, 64, 2369-2374.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









