Ti (IV) complexes of some heterocyclic ligands. Synthesis, characterization and ethylene polymerization activity
Hamdi Ali Elagab1,2
1Albaha University, Faculty of Science and Arts- Almandaq, Albaha, P.O. Box (1988),Saudi Arabia
2Laboratorium für Anorganische Chemie, Universität Bayreuth, Postfach 10 12 51, D-95440 Bayreuth, Germany
Corresponding Author Email: hamdieez2000@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320177
Article Received on :
Article Accepted on :
Article Published : 03 Feb 2016
31 complexes of bis - (benzimidazole, benzothiazole and benzoxazole) compounds with Ti (IV) metal centers were synthesized, characterized, activated with methylalumoxane (MAO) and then tested for catalytic ethylene polymerization. The activities of the various catalysts were found to be functions of the hetero atoms in the ligand frameworks. The highest activity was obtained with 39 / MAO (573 kg PE / mol cat. h). The produced polyethylenes showed high molecular weights (up to 1.5 ×106 g/mol) and broad molecular weight distributions (PD = 65). This could result from different interactions of the MAO counterion with the heteroatoms of the catalyst ligand generating different active sites.
KEYWORDS:benzimidazole; benzothiazole; benzoxazole; titanium complexes; bimodal resins
Download this article as:| Copy the following to cite this article: Elagab H. A. Ti (IV) complexes of some heterocyclic ligands. Synthesis, characterization and ethylene polymerization activity. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Elagab H. A. Ti (IV) complexes of some heterocyclic ligands. Synthesis, characterization and ethylene polymerization activity. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=13796 |
Introduction
A review of the literature revealed that 1,2-bis(2-benzothiazolyl)benzene and 1,2-bis(2-benzothiazolyl)ethane are frequently used as ligands and a considerable number of their complexes with late transition metals are reported1-6. Also, the nickel(II), cobalt(II) and copper(II) coordination chemistry of some tetradentate ligands involving benzothiazole functional groups has been published7. In all cases involving benzothiazoles as functional groups the ligands behave as nitrogen donors, except in a few cases involving bridging benzothiazole8, in which it is assumed to behave as a bidentate ligand involving both N and S donation. The ligand 2, 6-bis (2-benzothiazolyl) pyridine9, 10, has been shown to behave as an N-3donor in its complexes with manganese (II), iron (II) and nickel (II).2,6-Bis(benzimidazolyl)pyridine1, 11-23, and 2,6-bis (benzoxazolyl)pyridine24,derivatives have been reported as ligands for transition metals in order to investigate the complexes for their structures and properties.
In polyolefin chemistry, an increasing interest has been focused on the exploration and development of homogeneous transition metal catalysts, as a result of an increasing demand for polyethylene25-30. The vanadium complexes of bis(benzimidazole)amine tridentate ligands [N, N, N], were reported as active ethylene polymerization catalysts after activation with simple alkylaluminum compounds.[31] 2,6-Bis(2-benzimidazolyl)pyridine zirconium dichloride / MAO polymerizes methylacrylate.[32] Recently,[33- 39] we reported the ethylene polymerization activity of benzimidazole, benzothiazole, benzoxazole and 2-(benzimidazolyl) pyridine titanium zirconium and vanadium complexes. Herein we report on the effect of hetero atom on the activity of zirconium, complexes of bis (benzimidazole), benzothiazole, and benzoxazole), and their behaviour towards ethylene polymerization after activation with methylaluminoxane (MAO).
Results and Discussion
General synthesis of ligand precursors
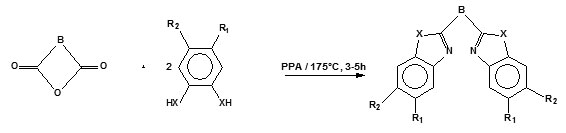
The condensation reaction of a dicarboxylic acid or an acid anhydride in preheated polyphosphoric acid is a well established procedure for the preparation of the imidazole based ligand precursors 20, 40, 41 in high yields (Scheme 1).
Scheme 1: Synthesis of ligand precursors 1-31.
|
No. |
Bridging unit (B) |
X |
R1 |
R2 |
|
1 |
– |
S |
H |
H |
|
2 |
– |
O |
H |
H |
|
3 |
– |
O |
CH3 |
H |
|
4 |
– |
NH |
H |
H |
|
5 |
– |
NH |
CH3 |
H |
|
6 |
CH2 |
S |
H |
H |
|
7 |
CH2 |
O |
H |
H |
|
8 |
CH2 |
O |
CH3 |
H |
|
9 |
CH2 |
O |
H |
CH3 |
|
10 |
CH2 |
NH |
H |
H |
|
11 |
CH2 |
NH |
CH3 |
H |
|
12 |
CH2 |
NH |
Cl |
H |
|
13 |
CH2CH2 |
S |
H |
H |
|
14 |
CH2CH2 |
O |
H |
H |
|
15 |
CH2CH2 |
O |
H |
CH3 |
|
16 |
CH2CH2 |
O |
CH3 |
H |
|
17 |
CH2CH2 |
NH |
H |
H |
|
18 |
CH2CH2 |
NH |
CH3 |
H |
|
19 |
CH2CH2 |
NH |
Cl |
H |
|
20 |
1,2-phenylene |
S |
H |
H |
|
21 |
1,2-phenylene |
O |
H |
H |
|
22 |
1,2-phenylene |
O |
CH3 |
H |
|
23 |
1,2-phenylene |
O |
H |
CH3 |
|
24 |
1,2-phenylene |
NH |
H |
H |
|
25 |
1,2-phenylene |
NH |
CH3 |
H |
|
26 |
1,2-phenylene |
NH |
Cl |
H |
|
27 |
4-Me-1,2-phenylene |
S |
H |
H |
|
28 |
4-Me-1,2-phenylene |
O |
H |
H |
|
29 |
4-Me-1,2-phenylene |
NH |
H |
H |
|
30 |
4-Me-1,2-phenylene |
NH |
CH3 |
H |
|
31 |
4-Me-1,2-phenylene |
NH |
Cl |
H |
Synthesis of titanium complexes 32-62
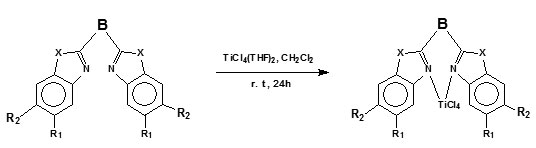
The complexes were synthesized according to Scheme 2. The titanium complexes were prepared by ligand displacement reactions. The reaction of the Tetrahydrofuran adducts of titanium tetrachloride with the corresponding ligand precursorin methylene chloride resulted in an immediate colour change and the complexes could be isolated in very high yields (80-95 %). The complexes were characterized by NMR, mass spectrometry and elemental analysis.
Scheme 2: Synthesis of the coordination compounds 32-62.
|
Complex No. |
Bridging unit (B) |
X |
R1 |
R2 |
|
32 |
– |
S |
H |
H |
|
33 |
– |
O |
H |
H |
|
34 |
– |
O |
CH3 |
H |
|
35 |
– |
NH |
H |
H |
|
36 |
– |
NH |
CH3 |
H |
|
37 |
CH2 |
S |
H |
H |
|
38 |
CH2 |
O |
H |
H |
|
39 |
CH2 |
O |
CH3 |
H |
|
40 |
CH2 |
O |
H |
CH3 |
|
41 |
CH2 |
NH |
H |
H |
|
42 |
CH2 |
NH |
CH3 |
H |
|
43 |
CH2 |
NH |
Cl |
H |
|
44 |
CH2CH2 |
S |
H |
H |
|
45 |
CH2CH2 |
O |
H |
H |
|
46 |
CH2CH2 |
O |
CH3 |
H |
|
47 |
CH2CH2 |
O |
H |
CH3 |
|
48 |
CH2CH2 |
NH |
H |
H |
|
49 |
CH2CH2 |
NH |
CH3 |
H |
|
50 |
CH2CH2 |
NH |
Cl |
H |
|
51 |
1,2-phenylene |
S |
H |
H |
|
52 |
1,2-phenylene |
O |
H |
H |
|
53 |
1,2-phenylene |
O |
CH3 |
H |
|
54 |
1,2-phenylene |
O |
H |
CH3 |
|
55 |
1,2-phenylene |
NH |
H |
H |
|
56 |
1,2-phenylene |
NH |
CH3 |
H |
|
57 |
1,2-phenylene |
NH |
Cl |
H |
|
58 |
4-Me-1,2-phenylene |
S |
H |
H |
|
59 |
4-Me-1,2-phenylene |
O |
H |
H |
|
60 |
4-Me-1,2-phenylene |
NH |
H |
H |
|
61 |
4-Me-1,2-phenylene |
NH |
CH3 |
H |
|
62 |
4-Me-1,2-phenylene |
NH |
Cl |
H |
Characterization
NMR spectroscopy
The ligand precursors were characterized by NMR spectroscopy using DMSO as solvent. Table 2 includes the 1H and 13C NMR data for compounds 1-31. For example, the 1H NMR spectrum of compound 15(Figure. 1) shows five signals, the doublet at δ =7.54ppm [d, 2H, JH,H = 8.1 Hz] assigned to H2, the signal at δ = 7.35ppm [s,2H] assigned to aromatic protons H1, the doublet at δ = 7.16ppm [d, 2H, JH,H = 8.1 Hz] corresponds to two protons H3. At δ = 3.54 ppm a singlet assigned to H4, and the signal upfield at δ = 2.50 ppm assigned to the s1x protons of the methyl groups.
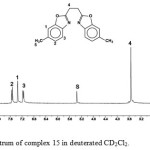 |
Figure 1: 1H NMR spectrum of complex 15 in deuterated CD2Cl2. Click here to View figure |
The 13C NMR spectrum of compound 15(Figure.2) shows nine signals. The two signals downfield at δ = 165.5 ppm and at δ = 149.4 ppmcorrespond to the carbon atoms 7 and 1 respectively. Each of the five signals at δ = 141.8, 134.43, 125.9, 119.7, and 109.9 ppm corresponds to two carbon atoms (6, 3, 4, 5 and 2 respectively) of the aromatic rings. The carbon atoms of the bridging group appear upfield at δ = 25.7 ppm. The methyl carbon appears at δ = 21.3 ppm
 |
Figure 2: 13C NMR spectrum of compound 15. Click here to View figure |
The 1H NMR spectrum of complex 46(Figure.3) shows four resonance signals. The doublet at δ = 7.52ppm [d, 2H, JH,H = 8.1 Hz] assigned to H2,, the signal at δ = 7.48 ppm[s, 2H,] can be assigned to H1,the doublet at δ = 7.14 ppm is assigned toH3. The bridging group protons H4 can be observed as a singlet at δ = 3.50 ppm. The singlet at δ = 2.42 ppm is assigned to the methyl protons H5.
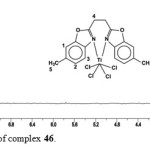 |
Figure 3: 1H NMR spectrum of complex 46. Click here to View figure |
The 13C NMR spectrum of complex 46 (Figure. 4) shows nine resonance signals each corresponding to two carbon atoms. The signals at δ = 167.2, 149.7, 142.3, 136.0, 127.6, 119.5, 111.2, 25.6 and 21.4 ppm can be assigned to the carbon atoms C7, C1, C6, C3, C4, C5, C2, C8, and C9 respectively.
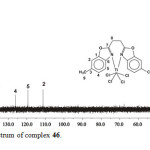 |
Figure 4: .3C NMR spectrum of complex 46. Click here to View figure |
The ligand precursors were also characterized by their mass spectra. The mass spectrum of compound 15 shows the molecular ion peak m/z = 292 and m/z = 146 (M°+-C9H8NO). The ion with the mass m/z = 132 can be assigned to (C8H6NO), (the full fragmentation pattern is shown in Figure 5).
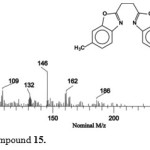 |
Figure 5: Mass spectrum of compound 15. Click here to View figure |
Figure 6 shows the mass spectrum of complex 46. The molecular ion peakm/z = 482. The ion with m/z = 449 represents the loss of one chloride, the ions with m/z = 411 result from the loss of two chlorides. The ion with m/z = 292 represents the mass of the free ligand.
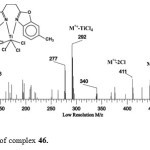 |
Figure 6: Mass spectrum of complex 46. Click here to View figure |
Elemental Analysis
The elemental analysis data of the synthesized ligands and their complexes are given in (Table 1). The data shows the formation of metal complexes in a 1: 1 (M: L) molar ratio.
Table 1: Elemental analysis data for heterocycles and their complexes.
|
Compound No. |
General Formula |
Calculated |
Found |
||||
|
C |
H |
N |
C |
H |
N |
||
|
1 |
C14H8N2S2 |
62.7 |
3.0 |
10.4 |
62.9 |
2.8 |
10.6 |
|
2 |
C14H8N2O2 |
71.2 |
3.4 |
11.9 |
70.8 |
3.6 |
12.1 |
|
3 |
C16H12N2O2 |
72.7 |
4.5 |
10.6 |
72.4 |
4.7 |
10.8 |
|
4 |
C14H10N4 |
71.8 |
4.3 |
23.9 |
71.6 |
4.5 |
24.1 |
|
5 |
C16H14N4 |
73.3 |
5.3 |
21.4 |
72.9 |
5.5 |
21.1 |
|
6 |
C15H10N2S2 |
63.8 |
3.5 |
9.9 |
63.6 |
3.4 |
10.2 |
|
7 |
C15H10N2O2 |
72.0 |
4.0 |
11.2 |
71.8 |
4.2 |
11.4 |
|
8 |
C17H14N2O2 |
73.4 |
5.0 |
10.1 |
73.6 |
5.1 |
9.8 |
|
9 |
C17H14N2O2 |
73.4 |
5.0 |
10.1 |
73.6 |
5.1 |
9.8 |
|
10 |
C15H12N4 |
72.6 |
4.8 |
22.6 |
71.8 |
4.6 |
22.4 |
|
11 |
C17H16N4 |
73.9 |
5.8 |
20.3 |
73.7 |
6.0 |
20.1 |
|
12 |
C15H10N4Cl2 |
64.9 |
4.1 |
9.5 |
65.2 |
4.4 |
9.1 |
|
13 |
C16H12N2S2 |
64.9 |
4.1 |
9.5 |
65.2 |
3.9 |
9.6 |
|
14 |
C16H12N2O2 |
72.7 |
4.6 |
10.6 |
72.9 |
4.5 |
10.5 |
|
15 |
C18H16N2O2 |
74.0 |
5.5 |
9.6 |
73.7 |
5.8 |
9.9 |
|
16 |
C18H16N2O2 |
74.0 |
5.5 |
9.6 |
73.8 |
5.7 |
9.6 |
|
17 |
C16H14N4 |
73.3 |
5.3 |
21.4 |
72.9 |
5.4 |
21.4 |
|
18 |
C18H18N4 |
74.5 |
6.2 |
19.3 |
74.8 |
5.9 |
19.6 |
|
19 |
C16H12N4Cl2 |
58.0 |
3.6 |
16.9 |
58.2 |
3.7 |
17.1 |
|
20 |
C20H12N2S2 |
68.8 |
3.5 |
8.1 |
69.7 |
3.5 |
8.2 |
|
21 |
C20H12N2O2 |
76.9 |
3.9 |
9.0 |
77.1 |
3.7 |
8.8 |
|
22 |
C22H16N2O2 |
77.7 |
4.7 |
8.2 |
77.7 |
4.7 |
8.4 |
|
23 |
C22H16N2O2 |
77.7 |
4.7 |
8.2 |
77.7 |
4.7 |
8.4 |
|
24 |
C20H14N4 |
77.4 |
4.5 |
18.1 |
77.4 |
4.5 |
18.1 |
|
25 |
C22H18N4 |
78.1 |
5.3 |
16.6 |
78.6 |
5.2 |
17.1 |
|
26 |
C20H12N4Cl2 |
63.3 |
3.2 |
14.8 |
64.1 |
3.5 |
15.2 |
|
27 |
C21H14N2S2 |
70.4 |
3.9 |
7.8 |
69.8 |
4.1 |
7.5 |
|
28 |
C21H14N2O2 |
77.3 |
4.3 |
8.6 |
76.9 |
4.6 |
8.9 |
|
29 |
C21H16N4 |
77.8 |
4.9 |
17.3 |
78.1 |
5.2 |
17.6 |
|
30 |
C23H20N4 |
78.4 |
5.7 |
15.9 |
78.1 |
5.5 |
16.2 |
|
31 |
C21H14N4Cl2 |
64.1 |
3.6 |
14.2 |
64.5 |
3.9 |
13.9 |
|
32 |
C14H8N2S2TiCl4 |
36.7 |
1.8 |
6.1 |
36.2 |
1.7 |
5.9 |
|
33 |
C14H8N2O2 TiCl4 |
39.4 |
1.9 |
6.6 |
39.8 |
1.8 |
6.7 |
|
34 |
C16H12N2O2 TiCl4 |
42.3 |
2.6 |
6.2 |
41.9 |
2.3 |
5.9 |
|
35 |
C14H10N4 TiCl4 |
39.6 |
2.4 |
13.2 |
39.1 |
2.6 |
13.7 |
|
36 |
C16H14N4 TiCl4 |
42.4 |
3.1 |
12.4 |
42.7 |
2.9 |
12.2 |
|
37 |
C15H10N2S2 TiCl4 |
38.1 |
2.1 |
5.9 |
38.4 |
2.1 |
5.6 |
|
38 |
C15H10N2O2 TiCl4 |
40.9 |
2.3 |
6.4 |
40.8 |
2.0 |
6.4 |
|
39 |
C17H14N2O2 TiCl4 |
42.7 |
2.9 |
5.9 |
42.3 |
3.0 |
5.4 |
|
40 |
C17H14N2O2 TiCl4 |
42.7 |
2.9 |
5.9 |
42.6 |
2.7 |
5.5 |
|
41 |
C15H12N4 TiCl4 |
41.9 |
2.8 |
13.0 |
42.2 |
2.6 |
13.4 |
|
42 |
C17H16N4 TiCl4 |
43.8 |
3.4 |
12.0 |
43.5 |
3.8 |
11.7 |
|
43 |
C15H10N4Cl2 TiCl4 |
35.5 |
2.0 |
11.0 |
35.2 |
2.1 |
10.8 |
|
44 |
C16H12N2S2 TiCl4 |
39.5 |
2.5 |
5.8 |
39.3 |
2.5 |
5.4 |
|
45 |
C16H12N2O2 TiCl4 |
42.3 |
2.6 |
6.2 |
42.0 |
2.2 |
5.9 |
|
46 |
C18H16N2O2 TiCl4 |
44.8 |
3.3 |
5.8 |
44.7 |
3.4 |
5.6 |
|
47 |
C18H16N2O2 TiCl4 |
44.8 |
3.3 |
5.8 |
45.2 |
3.1 |
5.9 |
|
48 |
C16H14N4 TiCl4 |
42.5 |
3.1 |
12.4 |
42.3 |
3.3 |
12.8 |
|
49 |
C18H18N4 TiCl4 |
45.0 |
3.8 |
11.7 |
45.6 |
3.5 |
11.4 |
|
50 |
C16H12N4Cl2 TiCl4 |
36.9 |
2.3 |
10.7 |
36.8 |
2.6 |
10.9 |
|
51 |
C20H12N2S2 TiCl4 |
44.9 |
2.2 |
5.2 |
45.3 |
1.9 |
4.9 |
|
52 |
C20H12N2O2 TiCl4 |
47.8 |
2.4 |
6.6 |
47.4 |
2.7 |
6.8 |
|
53 |
C22H16N2O2 TiCl4 |
49.8 |
3.0 |
5.3 |
49.7 |
2.9 |
5.5 |
|
54 |
C22H16N2O2 TiCl4 |
49.8 |
3.0 |
5.3 |
49.2 |
3.3 |
5.6 |
|
55 |
C20H14N4 TiCl4 |
48.0 |
2.8 |
11.2 |
48.5 |
2.4 |
11.5 |
|
56 |
C22H18N4 TiCl4 |
50.0 |
3.4 |
10.6 |
49.8 |
3.5 |
10.1 |
|
57 |
C20H12N4Cl2 TiCl4 |
42.3 |
2.1 |
9.9 |
42.5 |
1.9 |
9.5 |
|
58 |
C21H14N2S2 TiCl4 |
46.0 |
2.6 |
5.1 |
46.3 |
2.9 |
4.8 |
|
59 |
C21H14N2O2 TiCl4 |
48.8 |
2.7 |
5.4 |
47.9 |
3.0 |
5.2 |
|
60 |
C21H16N4 TiCl4 |
49.0 |
3.1 |
10.9 |
48.7 |
3.4 |
10.7 |
|
61 |
C23H20N4 TiCl4 |
50.9 |
3.7 |
10.3 |
51.3 |
3.4 |
10.6 |
|
62 |
C21H14N4Cl2TiCl4 |
43.3 |
2.4 |
9.6 |
43.7 |
2.6 |
9.4 |
Polymerization Results
All coordination compounds were activated with MAO according to the mechanism proposed for the activation of metallocene42, 43 and 2,6-bis(imino)pyridine iron (II)44 catalyst precursors.
The complexes of titaniumwith ligands derived from bis (benzoimidazolyl), bis (benzoxazolyl) and bis (benzothiazolyl) compounds showed variable activities for ethylene polymerization (Table 2).
Table 2: Polymerization activities of complexes 32-62.
All polymerization reactions were carried out in 250 ml pentane with MAO as cocatalyst (Al: M, 2500:1) at 50°C and 10 bar ethylene pressure).
Table 2: Polymerization activities of complexes 32-62.
| No. | Activity [kg/ mol cat. h] |
| 32 | 140 |
| 33 | 185 |
| 34 | 261 |
| 35 | 209 |
| 36 | 526 |
| 37 | 279 |
| 38 | 472 |
| 39 | 573 |
| 40 | 374 |
| 41 | 245 |
| 42 | 316 |
| 43 | 148 |
| 44 | 155 |
| 45 | 239 |
| 46 | 386 |
| 47 | 205 |
| 48 | 208 |
| 49 | 341 |
| 50 | 250 |
| 51 | 210 |
| 52 |
355 |
| 53 | 402 |
| 54 | 110 |
| 55 | 202 |
| 56 | 252 |
| 57 | 36 |
| 58 | 260 |
| 59 | 176 |
| 60 | 204 |
| 61 | 315 |
| 62 | 88 |
The activities are greatly influenced by the hetero atoms in addition to the ligand environment.
2,2-bis(benzimidazolyl), 1,1-Bis(benzimidazolyl)methane, 1,2-bis(benzimidazolyl)ethane and 1,2-bis(benzimidazolyl) benzene titanium complexes act as catalysts for ethylene polymerization, after activation with methylaluminoxane (MAO). They showed variable activities under different polymerization conditions.
The titanium complexes generally show moderate to good activities compared to the benchmark complexes zirconocene (Cp2ZrCl2).
The titanium complexes derived from the ligand systems 2, 2-bis (benzothiazolyl 32, benzoxazolyl (33, 34) and benzimidazolyl (35, 36) (Figure 7) shows the following activity order 35 /MAO>33 / MAO>32 / MAO.The methyl substituted complexes34 and 36 show higher activities compared to the unsubstituted complexes33 and 35. The different behaviours most probably occur due to the structure of the ligands in which the steric bulk and the electronic state greatly influence the activities of the complexes.
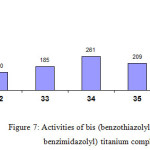 |
Figure 7: Activities of bis (benzothiazolyl, benzoxazolyl, benzimidazolyl) titanium complexes Click here to View figure |
Among the titanium complexes with 1, 1-bis (benzimidazolyl) methane ligands, the activities of the systems decrease in the order 41/MAO > 42/MAO>43/MAO (see Figure 8). This result is in contrast to the fact that complexes with both large steric bulk and high electron withdrawing effect will have the highest activities. Complex 43 witha chloro-substituentinmeta position to the imino nitrogen atoms shows a reduced activity, while complex 42 with a methyl group in the same position shows a somewhat higher activity. One explanation could be the fact that chlorine as a Lewis base can block the active sites of a neighbouring catalyst molecule.
The unsubstituted complex 41 shows the highest activity. The same trend is observed for the titanium complexes of 1, 2-bis (benzimidazolyl) benzene: 55/MAO>56/MAO>57/MAO.
For the titanium complexes derived from 1, 2-bis (benzimidazolyl) ethane, the activities decrease in the following order: 49/MAO>50/MAO>48/MAO(Figure 8).
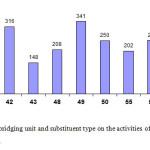 |
Figure 8: Effect of bridging unit and substituent type on the activities of bis (benzimidazolyl) titanium complexes. Click here to View figure |
Ethylene polymerization reactions with complexes of titanium with the ligand systems 1,1-bis(benzothiazolyl)methane, 1,2-bis(benzothiazolyl)ethane, and 1,2-bis(benzothiazolyl)benzene. The activities of titanium complexes decrease in the order of 1, 1-bis (benzothiazolyl)methane (37)>1,2-bis(benzothiazolyl)benzene (51)> 1,2-bis(benzothiazolyl) ethane (44) (Figure 9). This order of activity can be accounted for by structural variations of the ligand systems.
The activity of unsubstituted (benzothiazolyl, benzoxazolyl and benzimidazolyl) titanium complexes (Figure 9) shows great dependence on both heteroatom and bridging units. For example the complexes derived from the ligand systems bis(benzothiazolyl) shows the following order 1, 1-bis(benzothiazolyl) methane 37>1, 2- bis(benzothiazolyl) benzene 51>1, 2-bis(benzothiazolyl) ethane 44. The bis(benzoxazolyl, benzimidazolyl) derived titanium complexes shows the same activity order 1, 1-bis(benzoxazolyl) methane 38>1, 2- bis(benzoxazolyl) ethane 45>1, 2-bis(benzoxazolyl) benzene52.1, 1-bis (benzimidazolyl) methane 41>1, 2- bis (benzimidazolyl) ethane 48>1, 2-bis(benzimidazolyl) benzene55. The similar activity order of O, N containing ligands can be attributed to the similarities between the two hetero atoms.The 1,1-bis(benzoxazolyl) methane complex 38 shows a higher activity than the 1,2-bis(benzoxazolyl)benzene complex 52 and the 1,2-bis(benzoxazolyl)ethane complex 45. They show higher activities than those obtained from the benzothiazole ligand of the same type (Figure 9). This is most probably due to extra stabilization of the active species caused by the strong electronegative oxygen atom leading to an increase in electrophilicity of the metal center.
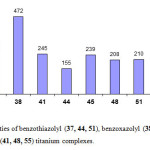 |
Figure 9: Activities of benzothiazolyl (37, 44, 51), benzoxazolyl (38, 45, 52) and benzimidazolyl (41, 48, 55) titanium complexes. |
The methyl substituted bis-(benzoxazolyl, and benzimidazolyl) titanium complexes (Figure 10)
Shows that, the bis benzoxazolyl complexes were more active than the bis benzimidazolyl complexes, for example the catalyst system 39 / MAO shows an activity of 573 [kg/ mol cat. h] while the catalyst derived from 1, 1, bis(benzimidazolyl) methane 42 / MAO shows an activity of 416 [kg/ mol cat. h].
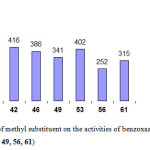 |
Figure 10: Effect of methyl substituent on the activities of benzoxazole (39, 46, 53) and benzimidazole (42, 49, 56, 61) Click here to View figure |
The methyl substituted bis-benzoxazole titanium complexes (Figure 11) behave differently depending on the position of the methyl group at the phenyl ring of the benzoxazolyl moiety. When the methyl group is introduced in the meta-position to the imino-nitrogen atom (39,46, 53) it leads to an increase of the activity due to both electronic and steric effects. On the other hand, when the methyl group is introduced in the para position (40, 47, 54) with respect to the imino nitrogen atom, the activities drop because the electron density on the nitrogen atom increases and consequently the nucleophilicity of the central metal atom increases which weakens the interaction between the metal atom and the p-electrons of the ethylene monomer and hence this decreases the rate of ethylene insertion in the chain-growth steps27, 45.
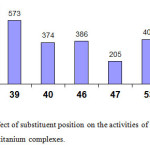 |
Figure 11: Effect of substituent position on the activities of benzoxazolyl titanium complexes. Click here to View figure |
The activities of bis (benzimidazolyl) benzene titanium complexes (55, 56, and 57)and bis (benzimidazolyl) – 4-methyl benzene titanium complexes (60, 61, and 62) (Figure 11) revealed that the chloro substituted complexes are more active than the methyl substituted complexes. However both chloro and methyl substituted complexes of bis (benzimidazolyl) benzene (56, 57) are much more active than bis (benzimidazolyl) – 4-methyl benzene titanium complexes (61, 62).
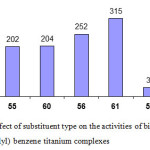 |
Figure 12: Effect of substituent type on the activities of bis (benzimidazolyl) benzene titanium complexes Click here to View figure |
GPC analysis of the polyethylenes produced by benzimidazole based complexes revealed that the symmetric catalysts systems were capable to produce resins with high to very high molecular weights associated with broad or even bimodal molecular weight distributions. The bimodality may arise from the fact that the MAO counterion induces the necessary dissymmetry of the active sites in the activation process46.
The molecular weight Mw of the polymer produced with 55 / MAO was determined to be 1.3× 106g / mol, (PD = 19.2) (see Figure 13).
 |
Figure 13: GPC profile for polyethylene produced with catalyst 55 / MAO. Click here to View figure |
The molecular weight Mw and polydispersity of the polymer produced with catalyst systems 35 / MAO, 41/ MAO, 49 / MAO and 58 / MAO was determined to be1.3 × 106 g / mol, (PD = 16.3), 1.1 × 106 g / mol, (PD = 8.4), 4.4.37 ×105 g / mol, (PD = 9.1), and 3.3 × 106 g / mol, (PD = 10.2) respectively.
Differential scanning calorimetric (DSC) measurements for representative samples of polyethylenes produced with bis-(benzimidazolyl, benzothiazolyl, and benzoxazolyl) zirconium complexes revealed that the catalyst systems were capable to produce high density polyethylenes with melting temperatures > 135 °C. The crystallization temperatures of the polymers range from 118 – 125 °C and the polymers have high degrees of crystallinities. For example, DSC curves for polyethylene produced with the catalysts 55 / MAO (see Figures 14) show melting temperatures 137.5 °C and crystallization temperatures of 120.3 °C.
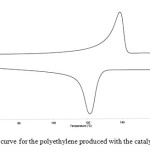 |
Figure 14: DSC curve for the polyethylene produced with the catalyst 55 / MAO. Click here to View figure |
Experimental
All experimental work was routinely carried out using Schlenk technique unless otherwise stated. Anhydrous and purified argon was used as inert gas. n-Pentane, diethyl ether, toluene and tetrahydrofuran were purified by distillation over Na/K alloy. Diethyl ether was additionally distilled over lithium aluminium hydride. Methylene chloride was dried with phosphorus pentoxide and additionally with calcium hydride. Methanol and ethanol were dried over magnesium. Deuterated solvents (CDCl3, DMSO-d6) for NMR spectroscopy were stored over molecular sieves (3Ǻ). Methylalumoxane (30 % in toluene) was purchased from Crompton (Bergkamen) and Albemarle (Baton Rouge, USA / Louvain – La Neuve, Belgium). Ethylene (3.0) and argon (4.8/5.0) were supplied by Rießner Company (Lichtenfels). All other starting materials were commercially available and were used without further purification. The titanium adducts were synthesized via published procedures 47.
NMR Spectroscopy
The spectrometer Bruker ARX 250 was available for recording the NMR spectra. The samples were prepared under inert atmosphere (argon) and routinely recorded at 25 °C. The chemical shifts in the 1H NMR spectra are referred to the residual proton signal of the solvent (d = 7.24 ppm for CDCl3, d = 2.50 ppm for DMSO-d6) and in 13C NMR spectra to the solvent signal (d = 77.0 ppm for CDCl3, d = 39.5 ppm for DMSO-d6).
Mass Spectrometry
Mass spectra were routinely recorded at the Zentrale Analytik of the University of Bayreuth with a VARIAN MAT CH-7 instrument (direct inlet, EI, E = 70 eV) and a VARIAN MAT 8500 spectrometer.
Gel permeation chromatography (GPC)
GPC measurements were routinely performed by SABIC Company (Riyadh, Saudi Arabia).
Elemental analysis
Elemental analyses were performed with a VarioEl III CHN instrument. The raw values of the carbon, hydrogen, and nitrogen contents were multiplied with calibration factors (calibration compound: acetamide).
General procedures for the syntheses of the complexes
Syntheses of organic compounds 1-31
A diamine compound (0.05mol) was mixed with a dicarboxylic acid or an acid anhydride (0.025mol) and the mixture was poured in 50 ml of preheated (100°C) polyphosphoric acid. The mixture was stirred and heated at 175°C for 3-5 hours. The reaction mixture was then poured into ice cold water and allowed to stand overnight. The precipitate was removed by filtration and washed several times with diluted sodium hydrogen carbonate solution and finally with water. The reaction product was then air dried and weighed. The products were characterized by NMR and mass spectrometry (Table 3) and elemental analyses (Table 32).
Titanium complexes 32-62
To 0.87g, (2.6 mmol) TiCl4 (THF) 2 in dichloromethane was added 2.6 mmol of the solid ligand. The reaction mixture was stirred over night at room temperature; the solid formed was collected by filtration under reduced pressure and washed several times with dichloromethane, then with pentane, dried in vacuo and weighed. The products were characterized by NMR and mass spectroscopy (Table 3), and by elemental analyses (Table 1).
Table 3: 1H NMR, 13C NMR and mass spectroscopic data of ligand precursors and complexes.
| No | 1H NMRd [ppm] | 13C NMR d [ppm] | Mass m/z (%) |
| 1 | 8.15(d,2H), 7.96(d,2H), 7.54(t,2H), 7.47(t,2H) | n.d. | 268 M°+ (100) |
| 2 | 7.93(d,2H), 7.73(d,2H), 7.51(t,4H) | 152.1, 151.2, 141.4, 127.8, 126.0, 121.8, 111.7 | 236 M°+ (100) |
| 3 | 7.63(s,2H), 7.11(d,2H), 6.98(d,2H)2.37(s,6H) | 155.4, 147.5, 141.3, 132.7,127.7,123.2,108.6, 20.9 | 264 M°+ (100) |
| 4 | 12.22(br,2H, NH), 7.11- 6.99(m,8H) | 156.0, 126.4,123.8, 116.0 | 234 M°+ (100) |
| 5 | 12.32(br,2H,NH), 6.99-6.96(m,2H), 6.87-6.84(m,4H), 2.23(s,6H,CH3) | 156.0, 155.6, 132.9, 126.1, 124.4, 123.2, 115.8, 115.6, 21.2 | 262 M°+ (100) |
| 6 | 8.04(d,2H), 7.95(d,2H), 7.47(t,2H), 7.43(t,2H), 5.05(s,2H,CH2) | 167.0, 153.2, 135.9, 127.0, 125.9, 123.2, 122.9, 38.5 | 282 M°+ (100) |
| 7 | 7.70(d,2H), 7.50 (d,2H), 7.31(t,4H), 4.62(s,2H, CH2) | 159.5, 151.5, 141.3, 125.5, 124.8, 120.4, 110.9, 29.6 | 250 M°+ (100) |
| 8 | 7.60(d,2H), 7.52(s,2H), 7.21(d,2H), 4.84(s,2H,CH2), 2.41(s,6H, 2CH3) | 161.4, 149.5, 141.5, 134.7, 126.9, 120.2, 110.9, 29.4, 21.6 | 278 M°+ (100) |
| 9 | 7.59(d,2H), 7.52(s,2H), 7.19(d,2H), 4.82(s,2H,CH2), 2.46(s,6H,2CH3) | 160.8, 151.5, 139.2, 136.0, 126.4, 119.7, 111.5, 29.3, 21.9 | 278 M°+ (100) |
| 10 | 12.41(s,2H, NH),7.46(m,4H),7.11(m,4H), 4.43(s,2H,CH2) | 150.8, 138.4 122.9, 115.4, 29.8 | 248 M°+ (100) |
| 11 | 7.32(d,2H), 7.23(s,2H), 6.91(d,2H), 4.35(s,2H,CH2), 2.33(s,6H,CH3) | 150.6, 139.2 137.9, 131.2, 123.5, 115.3, 114.7, 30.0, 21.9 | 276 M°+ (100) |
| 12 | 7.54(s,2H), 7.48(d,2H), 7.14(d,2H), 4.46(s,2H,CH2) | 149.3, 134.7, 132.5,129.6, 125.9, 116.6, 115.1, 26.8 | 317 M°+ (100) |
| 13 | 7.98(d,2H), 7.82(d,2H), 7.44(t,2H), 7.37(t,2H), 3.74(s,4H, 2CH2) | 169.6, 153.5, 135.5, 126.4, 125.3, 123.0, 121.9, 33.6 | 296 M°+ (100) |
| 14 | 7.65(t,2H), 7.46(t,2H), 7.28(d,4H), 3.56(s,4H) | 165.2, 151.2, 141.6, 125.2, 124.7, 120.2, 110.9, 25.7 | 264 M°+ (100) |
| 15 | 7.46(s,2H), 7.41(d,2H), 7.16(d,2H), 3.55(s,4H,2CH2), 2.47(s,6H,2CH3) | 165.5, 149.4, 141.8, 134.3, 125.9, 119.7, 109.9, 25.7, 21.3 | 292 M°+ (100) |
| 16 | 7.53(d,2H), 7.35(s,2H), 7.16(d,2H), 3.54(s,4H,2CH2), 2.50(s,6H,2CH3) | 164.8, 151.5, 139.3, 135.4, 125.5, 119.1, 110.7, 25.6, 21.6 | 292 M°+ (100) |
| 17 | 7.57 (d,4H), 7.26 (t,4H), 3.56(s,4H,2CH2) | 153.8, 135.4, 124.3, 114.6, 25.4 | 262 M°+ (100) |
| 18 | 7.31 (d,2H), 7.22(s,2H),) 6.90 (d,2H), 3.51 (s,4H,CH2), 2.33(s,6H,CH3) | 154.2, 138.7, 137.4, 131.2, 123.5, 115.0, 114.5, 27.0, 21.9 | 290 M°+ (100) |
| 19 | 7.50 (s,2H), 7.44(d,2H), 7.09(d,2H), 3.36 (s,4H,2CH2) | 156.2, 140.6, 138.0, 126.3, 122.1, 116.2, 115.1 27.0 | 331 M°+ (100) |
| 20 | 8.01(d,2H), 7.93-7.90(dd,2H), 7.77(d,2H), 7.61-7.58(dd,2H), 7.44(t,2H), 7.33(t,2H) | 166.4, 153.4, 136.6, 133.5, 131.6, 131.5, 127.2, 126.3, 123.8, 121.9 | 344 M°+ (100) |
| 21 | 8.07(t,2H), 7.81(t,2H), 7.72(t,2H), 7.53(d,2H), 7.35(d,4H) | 162.2, 151.0, 141.9, 132.5, 131.7, 127.4, 126.5, 125.5, 120.7, 111.5 | 312 M°+ (100) |
| 22 | 8.13(t,2H), 7.84(d,2H), 7.52(s,2H), 7.44(d,2H), 7.19(d,2H), 2.40(s,6H,2CH3) | 162.3, 149.4, 142.2, 134.9, 132.4, 131.6, 127.6, 127.4, 120.5, 110.9, 21.6 | 340 M°+ (100) |
| 23 | 8.12-8.09(m,2H), 7.85-7.82(m,2H), 7.60(d,2H), 7.37(s,2H), 7.20(d,2H), 2.41(s,6H, 2CH3) | 161.8, 151.4, 139.8, 136.5, 132.3, 131.7, 127.5, 126.7, 120.2, 111.4, 21.9 | 340 M°+ (100) |
| 24 | 7.88 (d,1H), 7.80(d,1H), 7.69(t,2H), 7.64(s,2H, N-H), 7.61(m,4H),7.26(m,4H), | 151.6, 137.9, 133.5, 131.9, 129.4, 123.5, 115.5 | 310 M°+(100) |
| 25 | 8.05(s,2H), 7.78(s,2H,N-H), 7.63(s,2H), 7.56(d,2H), 7.35(s,2H), 7.00(d,2H), 2.37(s,6H,CH3) | 151.6, 139.0, 137.8, 132.2, 132.0, 130.4, 129.9, 115.9, 115.1, 22.0 | 338 M°+ (100) |
| 26 | 8.14 (s,2H), 7.61 (br,4H), 7.55 (d,2H), 7.12(d,2H) | 154.3, 141.4, 138.9, 132.1, 130.4, 130.3, 126.6, 122.4, 117.1, 115.8 | 378 M°+ (100) |
| 27 | 8.01-7.95(m,2H), 7.84-7.79(m,3H), 7.74(s,1H), 7.48-7.44(m,3H), 7.38-7.33(m,2H), 2.50(s,3H, CH3) | 166.6, 166.5, 153.6, 153.5, 141.1, 136.8, 136.8, 133.5, 131.8, 131.2, 131.1, 131.0, 126.3, 126.2, 125.5, 125.4, 123.6, 123.5, 121.7, 121.6, 21.3 | 358 M°+ (100) |
| 28 | 7.97(d,1H), 7.89(s,1H), 7.64(d,2H), 7.59(d,1H), 7.49(d,2H), 7.31(t,4H), 2.37(s,3H,CH3) | 162.7, 162.6, 151.2, 151.1, 142.2, 142.1, 142.1, 132.1, 131.9, 131.19, 127.5, 125.5, 125.4, 124.9, 124.6, 124.5, 120.4, 120.2, 110.7, 110.6, 21.4 | 326 M°+ (100) |
| 29 | 9.67(s,2H,N-H), 7.97(d,1H), 7.92(s,1H), 7.56-7.51(m,4H), 7.48-7.46(d,1H), 7,18-7.15(m,4H), 2.40(s;3H,CH2) | 152.1,152.0,140.3, 132.6, 132.5, 131.1, 131.0 129.9, 127.3, 122.8, 122.7, 115.2, 21.5 | 324 M°+ (100) |
| 30 | 7.96(d,1H), 7.91(s,1H), 7.44-7.43(m,3H), 7.35(s,2H), 6.98(d,2H), 2.42(s,3H,CH3), 2.36(s,6H,2CH3) | 151.7,151.6,140.1, 132.5, 132.2, 132.1, 132.0, 131.0, 129.7, 127.2, 124.3, 124.2, 116.3, 115.5, 22.0, 21.4 | 352 M°+ (100) |
| 31 | 8.09(d,1H), 8.04(s,1H), 7.62(d,2H), 7.56-7.53(m,2H), 7.36(d,1H), 7.10(d,2H), 2.38(s,3H,CH3) | 155.0,155.1, 141.7,141.6, 139.2,139.1, 132.5,132.1, 130.8,130.3, 127.6,126.3,126.2 122.2,122.1 117.0,116.9, 115.7,115.6, 21.4 | 393 M°+ (100) |
| 32 | 8.23(d,2H), 7.97(d,2H), 7.56(t,2H), 7.50(t,2H) | n.d. | 458 M°+(20), 422 M°+-Cl (10), 387 M°+-2Cl (20), 317 M°+-4Cl (20), 268 M°+-TiCl4 (100) |
| 33 | 7.95(dd,4H), 7.60-7.56(m,4H) | 152.3, 150.9, 141.3, 128.5, 126.7, 121.8, 112.4 | 425 M°+(30), 390 M°+-Cl (25), 321 M°+-3Cl (10), 284 M°+-4Cl (20), 236 M°+-TiCl4 (100) |
| 34 | 7.66(s,2H), 7.20(d,2H),7.00(d,2H)2.39(s,6H) | 155.2, 147.7, 141.5, 132.6,127.5,123.0,109.1, 21.1 | 454 M°+(20), 418 M°+-Cl (10), 383 M°+-2Cl (10), 314 M°+-4Cl (20), 264 M°+-TiCl4 (100) |
| 35 | 12.50(br,2H,NH), 7.40(d,4H), 7.15(t,4H) | 156.1, 126.6, 123.8, 116.2 | 424°+(20), 352 M°+-2Cl (20), 317 M°+-3Cl (10),234 M°+-TiCl4 (100) |
| 36 | 12.12(br,2H,NH), 7.06(d,2H), 6.97(s,2H), 6.82(d,2H), 2.17(s,6H, 2CH3) | 156.1, 155.8, 133.0, 126.2, 124.6, 124.1, 115.9, 115.7, 21.3 | 495 M°+(10), 478 M°+-CH3 (15), 423 M°+-2Cl (20), 389 M°+-3Cl (20), 352 M°+-4Cl(20) 262 M°+-ZrCl4 (50) |
| 37 | 8.02(d,2H), 7.92(d,2H), 7.45(t,2H), 7.38(t,2H), 5.02(s,2H,CH2) | 167.0, 153.2, 135.8, 127.0, 125.9, 123.2, 122.9, 38.4 | 472 M°+(20), 436 M°+-Cl (15), 401 M°+-2Cl (10), 365 M°+-3Cl (25), 282 M°+-TiCl4 (100) |
| 38 | 7.69(d,4H), 7.36(t,4H), 4.85(s,2H,CH2) | 161.4, 151.2, 141.3, 126.1, 125.4, 120.4, 111.5, 29.3 | 438 M°+(20), 368 M°+-2Cl (10), 331 M°+-3Cl(10), 295 M°+-4Cl (15), 250 M°+-TiCl4 (100) |
| 39 | 7.61(d,2H), 7.53(s,2H), 7.23(d,2H), 4.84(s,2H,CH2), 2.41(s,6H,2CH3) | 161.5, 149.4, 141.5, 134.8, 127.0, 120.2, 111.0, 29.4, 21.7 | 468 M°+(15), 432 M°+-Cl (20),361 M°+-3Cl (30), 296 M°+-4Cl-2CH3 (25), 278 M°+-TiCl4 (100) |
| 40 | 7.60(d,2H), 7.54(s,2H), 7.21(d,2H), 4.82(s,2H,CH2), 2.44(s,6H,2CH3) | 160.8, 151.5, 139.1, 136.1, 126.5, 119.8, 111.5, 29.3, 21.9 | 468 M°+(20), 432 M°+-Cl (10), 361 M°+-3Cl (20), 296 M°+-4Cl-2CH3 (20), 278 M°+-TiCl4 (100) |
| 41 | 7.52-7.49(m,4H), 7.52-7.14(m,4H), 4.55(s,2H,CH2) | 149.0, 135.4, 124.4, 115.1, 27.5 | 438 M°+(10), 402 M°+-Cl (20), 367 M°+-2Cl (20), 248 M°+-TiCl4 (100) |
| 42 | 7.63(d,2H), 7.54(s,2H), 7.31(d,2H), 5.25(s,2H,CH2), 2.42(s,6H,2CH3) | 146.3, 136.3, 132.3, 130.1, 127.8, 114.3, 114.1, 25.8, 21.8 | 466 M°+(10), 428 M°+-Cl(15), 393 M°+-2Cl (15), 357 M°+-3Cl (20), 319 M°+-4Cl (20), 276 M°+-TiCl4 (100) |
| 43 | 7.88(s,2H), 7.80(d,2H), 7.51(d,2H), 5.18(s,2H,CH2) | 149.3, 134.7, 132.6, 129.8, 125.9, 116.5, 114.9, 26.9 | 506 M°+(10), 470 M°+-Cl (20), 434 M°+-2Cl (15), 397 M°+-3Cl (10), 316 M°+-TiCl4 (100) |
| 44 | 8.02(d,2H), 7.99(d,2H), 7.44-7.35(m,4H), 3.68(s,4H,2CH2) | 170.6, 153.3, 135.3, 126.9, 125.8, 123.1, 122.9, 33.1 | 486 M°+(10), 450 M°+-Cl (15), 415 M°+-2Cl (10),296 M°+-TiCl4 (75) |
| 45 | 7.59(t,2H), 7.26(t,2H), 7.16(d,2H), 7.09(d,2H), 3.47(s,4H,2CH2) | 166.1, 150.9, 141.4, 125.5, 124.9, 112.0, 111.2, 25.3 | 454 M°+(20), 418 M°+-Cl (15), 381 M°+-2Cl (20), 312°+-4Cl (25), 264 M°+-TiCl4 (100) |
| 46 | 7.53(s,2H), 7.48(d,2H), 7.24(d,2H), 3.76(s,4H,2CH2), 2.39(s,6H,2CH3) | 167.2, 149.7, 142.3, 136.0, 127.6, 119.5, 111.2, 25.6, 21.4 | 486 M°+(20), 419 M°+-Cl-2CH3 (10),414 M°+-2Cl (15), 378 M°+-3Cl (30),296 M°+-TiCl4 (100) |
| 47 | 7.52(s,2H), 7.49(d,2H), 7.14(d,2H), 3.49(s,4H,2CH2), 2.42(s,6H,2CH3) | 165.5, 151.2, 139.3, 135.4, 126.0, 119.4, 111.3, 25.3, 21.9 | 486 M°+(20), 449 M°+-Cl (15) 421 M°+-Cl-2CH3 (15),414 M°+-2Cl (20), 378 M°+-3Cl (20),296 M°+-TiCl4 (100) |
| 48 | 7.76-7.70(m,4H), 7.50-7.43(m,4H), 3.95(s,4H,2CH2) | 152.5, 132.6, 125.6, 114.6, 24.5 | 452 M°+(10), 416M°+-Cl (20), 380 M°+-2Cl (15), 343 M°+-3Cl (10), 310 M°+-4Cl (20), 262 M°+-TiCl4 (10) |
| 49 | 7.62(d,2H), 7.53(s,2H), 7.30(d,2H), 3.87(s,4H,2CH2), 2.46(s,6H,2CH3) | 151.1, 136.3, 133.0, 130.0, 126.7, 114.7, 114.4, 24.7, 21.9 | 480 M°+(20), 443 M°+-Cl (20), 408 M°+-2Cl 1(5), 372 M°+-3Cl (15), 336 M°+-4Cl (20), 290 M°+-TiCl4 (100) |
| 50 | 7.84(s,2H), 7.78(d,2H), 7.50(d,2H), 3.78(s,4H,2CH2) | 154.3, 135.5, 133.6, 130.3, 125.0, 116.2, 115.0, 25.7 | 518 M°+(10), 485 M°+-Cl (15), 450 M°+-2Cl (10), 413 M°+-3Cl (10),377 M°+-4Cl (20) 330 M°+-TiCl4 (100) |
| 51 | 8.05(d,2H), 7.97-7.94(m,4H), 7.77-7.74(dd,2H), 7.50(t,2H), 7.44(t,2H) | 166.4, 153.4, 136.6, 133.5, 131.7, 131.6, 127.3, 126.4, 123.8, 123.0 | 534 M°+(10), 498 M°+-Cl (20), 463 M°+-2Cl (20), 427 M°+-3Cl (10), 344 M°+-TiCl4 (100) |
| 52 | 8.11-8.09(dd,2H), 7.83-7.81(dd,2H), 7.68(t,2H), 7.53(t,2H), 7.35-7.33(m,4H) | 162.2, 151.0, 141.9, 132.5, 131.7, 127.4, 126.5, 125.5, 120.7, 111.5 | 545 M°+(10),509 M°+-Cl (20), 472 M°+ -2Cl (15), 312 M°+ -ZrCl4 (100) |
| 53 | 8.12(d,2H), 7.85(t,2H), 7.53(s,2H), 7.46(d,2H), 7.21(d,2H), 2.42(s,6H,2CH3) | 162.3, 149.3, 142.2, 134.9, 132.4, 131.6, 127.5, 127.4, 120.5, 110.9, 21.6 | 530 M°+(10),464 M°+-Cl-2CH3 (20),459 M°+-2Cl (15), 423 M°+ -3Cl (15), 388 M°+-4Cl (10), 340 M°+– TiCl4 (100) |
| 54 | 8.14-8.11(dd,2H), 7.87-7.84(dd,2H), 7.62(d,2H), 7.41(s,2H), 7.22(d,2H), 2.43(s,6H,2CH3) | 161.8, 151.4, 139.8, 136.6, 132.4, 131.7, 127.5, 126.7, 120.2, 111.5, 21.9 | 530 M°+(15),494 M°+-Cl (20),430 M°+-2Cl-2CH3 (15), 423 M°+ -3Cl (10), 389 M°+-4Cl (20), 340 M°+– TiCl4 (100) |
| 55 | 8.37(d,2H), 8.08(t,2H), 7.924-7.835(m,4H), 7.637-7.625(m,4H) | 148.2, 134.0, 133.8, 133.6, 126.5, 125.1, 115.4 | 500 M°+(10), 463 M°+-Cl (20), 428 M°+-2Cl (15), 394 M°+-3Cl (15), 357 M°+-4Cl (20), 310 M°+-TiCl4 (100) |
| 56 | 8.18-8.16(dd,2H), 7.92-7.90(dd,2H),7.54(d,2H), 7.44(s,2H), 7.24(d,2H), 2.40(s,6H,2CH3) | 147.7, 137.6, 133.2, 131.9, 129.6, 128.9, 127.6, 126.1, 114.8, 114.5, 21.8 | 530 M°+(20), 492 M°+-Cl (10), 454 M°+-2Cl (15), 418 M°+-3Cl (20), 338 M°+-TiCl4 (50) |
| 57 | 8.22(d,2H), 7.92(t,2H), 7.74(s,2H), 7.69(d,2H), 7.43(d,2H) | 151.0, 136.6, 134.5, 132.4, 132.1, 129.3, 126.6, 125.3, 116.8, 115.1 | 568 M°+(10), 481 M°+-2Cl-NH (20), 462 M°+-3Cl (10), 426 M°+-4Cl (20), 378 M°+-TiCl4 (100) |
| 58 | 8.02(t,2H), 7.92(t,2H), 7.82(d,1H), 7.73(s,1H), 7.53(d,1H), 7.49-7.43(dd,2H), 7.41-7.35(dd,2H), 2.47(s,3H,CH3)- | 166.5, 166.4, 153.4, 153.4, 141.6, 136.6, 136.5, 133.5, 132.1, 132.0, 131.6, 130.8, 127.1, 127.2, 126.3, 126.2, 123.8, 123.7, 122.9, 122.8, 21.5 | 548 M°+(10), 512 M°+-Cl (20), 496 M°+-Cl-CH3 (15), 441 M°+-3Cl (20),405 M°+-4Cl (20), 358 M°+-TiCl4 (100) |
| 59 | 7.98(d,1H), 7.91(s,1H), 7.68-7.64(m,2H), 7.61(d,1H), 7.53-7.49(dd,2H), 7.32(t,4H), 2,45(s,3H,CH3) | 162.3, 162.3, 151.0, 150.9, 142.8, 141.9, 141.8, 133.0, 132.1, 131.6, 127.3, 126.4, 126.3, 125.5, 125.4, 124.6, 120.7, 120.6, 111.5, 111.4, 21.5 | 516 M°+(10),480 M°+-Cl (10),444 M°+-2Cl (20), 408 M°+ -2Cl(10), 372 M°+-4Cl (20), 326 M°+– TiCl4 (100) |
| 60 | 8.13(d,1H), 7.70-7.67(m,4H), 7.45-7.44(m,6H), 2.51(s,3H,CH3) | 149.2, 143.1, 135.1, 134.5, 133.1, 132.7, 126.0, 125.5, 125.3, 122.9, 115.3, 115.2, 21.6 | 516 M°+ (20), 443 M°+-2Cl (20), 409 M°+-3Cl (20), 372 M°+-4Cl (20), 324 M°+-TiCl4 (50) |
| 61 | 8.27(d,2H), 7.92(d,1H), 7.74(d,2H), 7.63(s,2H), 7.45(d,2H), 2.65(s,6H,2CH3), 2.61(s,3H,CH3) | 147.0, 146.9, 144.3, 136.6, 136.5, 134.7, 134.1, 133.6, 133.5, 131.6, 131.4, 129.9, 129.2, 128.1, 126.3, 124.2, 121.5, 114.8, 114.7, 114.5, 22.1, 21.9 | 542 M°+ (20), 468 M°+-2Cl (20), 433 M°+-3Cl (15), 371 M°+-4Cl -2CH3 (20), 352 M°+-TiCl4 (100) |
| 62 | 8.06(s,1H), 8.04 (s,2H) 7.70-7.61(m,4H), 7.39-7.34(m,2H), 2.45(s,3H,CH3) | 151.2, 142.7, 137.0, 136.3, 134.8, 134.1, 132.7, 132.5, 132.1, 132.1, 126.8, 125.9, 125.3, 125.1, 123.5, 116.7, 116.6, 115.2, 115.0, 21.5 | 582 M°+(20), 565 M°+-CH3 (15), 545 M°+-Cl (20), 508 M°+-2Cl (20), 472 M°+-3Cl (25), 458 M°+-4Cl-CH3 (15), 393 M°+-TiCl4 (100) |
Polymerization of ethylene
An amount of 2 – 5 mg of the desired complex was suspended in 5 ml of toluene. Methylaluminoxane (30% in toluene) was added resulting in an immediate color change. The mixture was added to a 1l Schlenk flask filled with 250 ml n-pentane. This mixture was transferred to a 1l Büchi laboratory autoclave under inert atmosphere and thermostated.An ethylene pressure of 10 bar was applied for one hour. The polymer was filtered over a frit, washed with diluted hydrochloric acid, water, and acetone, and finally dried in vacuo.
Conclusions
Mononuclear transition metal complexes with hetero atoms in the ligand system can offer a big advantage. After activation with MAO, more than one active center can be generated and as a consequence bimodal or multi modal resins are produced in catalytic ethylene polymerization reactions. Obviously the hetero atoms of the activated catalysts undergo different interactions with the Lewis acidic moieties of MAO. Structure-property-relationship studies indicate that the nature of the hetero atom and steric and electronic conditions in the coordination sphere are strongly influence the performance of such catalysts.
Acknowledgements
The author thanks SABIC Company (Riyadh, Saudi Arabia) for GPC measurements and University of Bayreuth (Germany) for the laboratory facilities.
References
- Lever, A. B. P.; Ramaswamy, B. S.; Simonsen, S. H.; Thompson, L. K. Can. J. Chem. 1970, 48, 3076- 3088.
CrossRef - RendellJ. C.; Thompson, L. K. Can. J. Chem.1979, 57,1-7.
CrossRef - Thompson, L.K.; Rendell, J. C.; Wellon, G.C. Can. J. Chem.1982, 60, 514-520.
CrossRef - Duff, E. J.; Hughes M. N.; Rutt, K. J. J. Chem. Soc. A.1969, 2101-2105.
CrossRef - Campell, M. J. M.; Card, D. W.; Grzeskowiak, R.; Goldstein M. J. Chem. Soc. A, 1970, 672-675.
CrossRef - Chan, N. N. Y.; Goodgame, M.; Weeks, M.J. J. Chem. Soc. A.1968, 2499-2501.
CrossRef - Thompson, L. K.; Ball, R. G.; Trotter, J. Can. J. Chem.1980, 58, 1566-1576.
CrossRef - Duff, E. J.; Hughes, M. N.; Rutt, K. J. J. Chem. Soc. A.1968, 2354-2357.
CrossRef - Livingstone, S.E.; Nolan, J. D.J. Chem. Soc. Dalton Trans.1972, 218-223.
CrossRef - Salameh, A. S.; Tayim, H. A. Polyhedron 1982, 6, 543-548.
CrossRef - Wang, S.; Cui, Y.; Tan, R.; Luo, Q.; Shi, J.; Wu, Q. Polyhedron1994, 11, 1661–1668.
CrossRef - Wang, J.; Zhu, Y.; Wang, S.; Gao, Y.; Shi, Q. Polyhedron1994, 13, 1405-1409.
CrossRef - Holz, R. C.; Thomson, L. C. Inorg. Chem. 1988, 27, 4640 -4644.
CrossRef - Wang, S. X.; Zhu, Y.; Zhang, F. G.; Wang, Q. Y.; Wang, L. F.Polyhedron1992, 11, 1909-1915.
CrossRef - Addison, A. D.; Burman, S.; Wahlgren, C. B.; Raijan, O. A.; Rowe, T. W.; Sinn, E., J. Chem. Soc. Dalton Trans. 1987, 2621 – 2630.
CrossRef - Ruttimann, S.; Moreau, C. M.; Williams, A. F.; Bernardinelli, G.; Addison, A. W. Polyhedron1992, 11, 635-646.
CrossRef - Nelson, S. M.; Esho, F. S.; Drew, M. G. B. J. Chem. Soc. Dalton Trans. 1982, 407-415.
CrossRef - Shashikala, N.; Gayathri, V.; Gowda, N. M. N.; Reddy, G. K. N. J. Indian Chem. Soc. 1989, 66, 537–540.
- Sanni, S. B.; Behm, H. J.; Benrskens, P. T.; Albada, G. A. V.; Reedijk, J.; Lenstra, A. T.H.; Addison, A. W.; Palaniandavar, M. J. J. Chem. Soc. Dalton Trans. 1988, 1429-1435.
CrossRef - Wellon, G. C.; Bautista, D. V.; Thompson, L. K.; Hartstock, F. W. Inorg. Chim.Acta.1983, 75, 271-276.
CrossRef - Breslow, R.; Hunt, J.T.; Smiley, R.; Tamowski, T. J. Am. Chem. Soc. 1983, 105, 5337 -5342.
CrossRef - Beer, R. H.; Tolmann, W. B.; Bott, S. G.; Lippard, S. J. Inorg. Chem. 1989, 28, 4557–4558.
CrossRef - Winter, J. A.; Caruso, D.; Shepherd, R. E. Inorg. Chem. 1988, 27, 1086-1089.
CrossRef - Jin, G.; Chen, F. CN 101089022A (2007).
- Britovsek, G. J. P.; Gibson, V. C.; Wass, D. F. Angew. Chem. Int. Ed. Engl. 1999, 38, 428-447.
CrossRef - Ittel, S. D.; Johnson, L. K.; Brookhart, M. Chem. Rev. 2000, 100, 1169-1204.
CrossRef - Zhang, W.; Sun, W.-H.; Zhang, S.; Hou, J.; Wedeking, K.; Schultz, S.; Fröhlich, R.; Song, H. Organometallics2006, 25, 1961-1969.
CrossRef - Kaminsky, W.; Fernandes, M. Polyolefins2015, 2, 1-19.
- Redshaw, C.; Tang, Y. Chem. Soc. Rev. 2012, 41, 4484 – 4510.
CrossRef - Baier, M.C.; Zuideveld, M.A.; Mecking, S. Angew. Chem. Int. Ed.2014, 53, 9722- 9744
CrossRef - Tomov, A. K.; Gibson, V. C.; Zaher, D.; Elsegood, M. R. J.; Dale, S. H. Chem. Commun. 2004, 1956-1957.
CrossRef - Cho, H. Y.; Tarte, N. H.; Cui, L.; Hong, D. S.; Woo, S. I.; Gong, Y.-D. Macromol. Chem. Phys. 2006, 207, 1965-1971.
CrossRef - Elagab, H. A.; Alt, H. G. Inorg. Chim.Acta.2015, 428, 100 -111.
CrossRef - Elagab, H. A.; Alt, H. G. Eur. Poly. J. 2015, 68, 385- 397.
CrossRef - Elagab, H. A.; Alt, H. G. Inorg.Chim.Acta.2015, 431,266 – 275.
CrossRef - Elagab, H. A.; Alt, H. G. Jordan Journal of Chemistry 2015, 10, 1-19.
CrossRef - Elagab, H. A.; Alt, H. G. Jordan Journal of Chemistry2015, 10, 41-57.
CrossRef - Elagab, H. A.; Alt, H. G. Eur. Poly. J.2015, 71, 85- 98.
CrossRef - Elagab, H. A.; Alt, H. G. Inorg.Chim. Acta.2015, 437, 26 – 37.
CrossRef - Phillips, M. A.J. Chem.Soc. 1928, 2393-2399.
CrossRef - Rai, C.; Braunwarth, J. B. J. Org. Chem. 1961, 26, 3434 – 3436.
CrossRef - Bochmann, M.; Wilson, L. M. J. Chem. Soc. Chem. Commun. 1986, 21, 1610-1611.
CrossRef - Jordan, R. F.; Bajgur, C. S.; Dasher, W. E.; Rheingold A. L. Organometallics1987, 6, 1041-1051.
CrossRef - Seitz, M.; Milius, W.; Alt, H. G. J. Mol. Catal.A. 2007, 261, 246-153.
CrossRef - Oouchi, K.; Mitani, M.; Hayakawa, M.; Yamada, T. Macromol. Chem. Phys. 1996, 197, 1545.
CrossRef - Yang, M; Yan, Wei-d.;Shen, Xiao-I.; Mu,B.; Wang, Bai-q. Chinese J. Poly. Sci.2008,26, 415-423.
CrossRef - Manzer, L. E., Inorganic Synthesis1982, 21, 135-140.

This work is licensed under a Creative Commons Attribution 4.0 International License.









