Synthesis of Some Novel Compounds of Saccharinyl Acetic Acid Containing Nucleus and Evaluation of Their Biological Activities as Antimicrobial
Magda H. Abdellattif
Pharmaceutical Chemistry Department, Pharmacy College, Deanship of Scientific Research, Taif University, Taif, KSA Corresponding author email: magdah11uk@hotmail.com
DOI : http://dx.doi.org/10.13005/ojc/320164
Article Received on :
Article Accepted on :
Article Published : 18 Feb 2016
A new series of Compounds of Saccharinyl Acetic acid Containing nucleus have been prepared via an improved synthetic procedure. Where saccharinyl moiety have been introduced to 4-benzylidine-2-methyl-1,3-oxazole-5-one in position 2 , compound (3) which has been reacted with nitrogen neucleophiles as hydrazine hydrate , phenyl haydrazine, aniline, p-toludine, m,p-aminobezoic acid to get compounds from (4-6). Also the reaction of compound (3) witharomatic substrate in presence of anhydrous AlCl3 (Friedel – Crafts reaction) afforded acetamide derivative (7) via the elimination of arylidine group. Moreover saccharinyl acetic acid hydrazide (8) was refluxed in acetic anhydride to give benzisothiazole derivative (9), which reacted with carbon nuleophiles (Grignard reagent) to afford compound (10). But when compound (9) reacted with PCl5/POCl3 it gave compound (11) which reacted with urea and thiourea to give compound (12(a, and b)). Also the condensation of compound (9) with aromatic aldehyde gave compound (13). Structures of all synthesized compounds were elucidated from I.R., 1HNMR, mass-spectroscopy, and elemental analysis.
KEYWORDS:Saccharin; hydrazinehydrate; oxazole; imidazole; benzisothiazole
Download this article as:| Copy the following to cite this article: Abdellattif M. H. Synthesis of Some Novel Compounds of Saccharinyl Acetic Acid Containing Nucleus and Evaluation of Their Biological Activities as Antimicrobial. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Abdellattif M. H. Synthesis of Some Novel Compounds of Saccharinyl Acetic Acid Containing Nucleus and Evaluation of Their Biological Activities as Antimicrobial. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14026 |
Introduction
The reported pharmaceutical properties of saccharin and its derivatives1-4 and in connection5-9 with our ongoing interest to developing new synthetic strategies for construction of heterocyclic systems involving saccharin due to its significant biological10-12 and pharmacological activities. Also imidazole derivatives are of interest to medical chemists for many years because of their biological activities such as anticancer, anti tubercular, antibacterial, and antifungal activities13-22 In view of the above facts we report in the present work the synthesis and antimicrobial activities of Saccharinyl derivatives.
Methods and Experiments
A-Chemistry
Melting points were determined in open capillaries using Gallen Kamp melting point apparatus and are uncorrected F.T.-IR spectra (KBr.disc) were recorded on a Perkin Elmer 1720 F.T. – IR spectrometer. 1H – NMR spectra were recorded on varin Gemini NMR spectrometer 300 MHz using TMS as internal standard. All reactions were monitored by TLC using aluminum silica gel plates 60 F 245. Elemental analysis and antimicrobial activity were carried out at micro-analytical center, faculty of science, cairo university, Egypt. Elemental analysis of all synthesized compounds is in agreement with the structure elucidated.
Benzylidene-2-Saccharinyl methyl-1,3-Oxazole-5-one.(3)
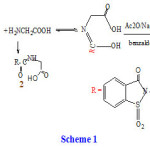
A mixture of Saccharinyl acetic acid chloride (10mmol) and glycine (10mmol) were refluxed in boiling ethanol for 1hr. The spectral solid was then refluxed for 4 hr. in freshly distilled acetic anhydride (30ml) and anhydrous sodium acetate in the presence of benzaldehyde. The reaction mixture was poured onto water and solid that separated was re-crystallized from ethanol to give (3). Orange (68%) mp 140-142 0C, IR (KBr, Cm-1) : υ 1700 (10 azlactone), 1610 (CN) , 1337 and 1120 (SO2). EI – MS: m/z 368 [M+]. Analytical calculation for C18H12O5N2S; C58.7;H3.3; N7.6 found C58.65; H3.3; N, 7.55.28,30
5-Benzylidene–3–saccharinyl methyl-1, 2, 4–triazin–6–one (4a,4b)
A mixture of 3 ( 10mmol) and hydrazine hydrate or phenyl hydrazine ( 10 mm ol) in absolute ethanol (30 ml) was refluxed for 3hr. the solid that obtained was recrystallized from ethanol to afford 4a: orange ( 75%), mp 163 -165oC ; IR (KBr, cm-1): 1700 (CO) , 1615 ( C=N) , 3350-3450 (br NH – NH) and 1337 and 1138 ( SO2) MF C18 H14 O4 N4.S EI –MS : m/z 382 (M+). And 4b : brown ( 80%), mp 95 -97 ; IR (KBr, cm-1) : 1680 ( CO) , 1610 ( C=2), 3250 (NH), 1330 and 1168 (SO2) MF C24H18O4N4S. EI- MS: m/z 460 (M+)
General procedure for the preparation of cinnamamide derivatives ( 5a-d) and imidazole one derivatives (6a-d).
A mixture of 3(10 mml ol) and aromatic amine oraminobenzoicacid ) was reflexed for 3h. in absolute ethanol (30 ml).
For compounds (5a-d) and in n-nutanol (30ml) for compounds (6a-d). The solid that obtained was recrystallized from proper solvent to afford (5a-d) and / or (6a-d).30
(α-Saccharinyl- N- acetamido) cinnamamide (5a)
Yellowish (70%) ,mp 210 -212 ; IR( KBr, cm-1) : 1670 and 1660 (CO), 3300-3200 (NH), 1330 and 1170 (SO2) . MF C24 H19 O5 N3S’ El- MS : m/z 461 (M+) , crystallized from benzene (5b) dark brown (75%) , mp 219-221; IR( KBr cm-1).
1670 (CO), 3320, 3250 (NH) 1616 (C=N), 1320 and 1170 (SO2).
MF C25H21O5N9S, El- MS: m/z 476 (M+1) recrystallized from toluene.
(5c) yellow (65%) ,mp 90-92 , IR(5Br, cm-1):1720 , ( CO) , 1680 , (CO amide), 3350-3400 (broud NH, OH) , 1310 and 1120 (SO2). MF C25H19O7N3S, EI –MS:
m/z 505 (M+), crystallized from benzene.
(5d) brown ( 70%) , mp 110-112 , IR ( KBr, cm-1):1710 (CO), 1670 ( CO amide) , 3300-3450(broud NH, OH), 1300 and 1110 (SO2).
MF C25H19O7N3S. EI – MS : m/z 505 (M+). recrystallized from benzene.
(6a) brown (75%) ,mp 180-182, IR( KBr, cm-1):1660 (CO) , 1600 ( CO amidazolone) 1580 (C=N).1330 and 1160 (SO2). MF C24 H17 O4 N3 S EI- MS : m/z 443 (M+) recrystallized from toluene.
(6b). brown (65%) ,mp 202-204 , IR(KBr, cm-1):
1670 (CO), 1630 ( CO imidazole), 1580( C=N), 1320 and 1170 (SO2).
M.F. C25H19O4N3S. EI- MS: m/z 457 (M+).
(6c) brown (70%), mp 150-152 , IR (KBr , cm-1) , 1680 (coimidazolone) , 1610 (C=N), 1320 and 1110 ( SO2) , 3450 (OH). MF C25 H17O6N3S EI-MS: m/z 487 (M+), recrystallized from benzene.
(6d) yellowish (77%) ,mp 155-157, IR (KBr, cm-1) : 1690 (Coimidazolone) , 1620 (C=N), 1280 and 1120 (SO2) 3440 (OH).
M.F C25H17O6N3S. EI- MS : m/z 487 (M+) recrystallized from toluene.
General procedure for the preparation of imidazolone derivatives ( 6a-d) From ( saccharinyl- N- acetamido ) cinnamide derivatives( 5a-d) by cyclization .
A solution of compounds 5a-d (10 m mole) in acetic anhydride (15ml) was boiled under reflux for 2h. The resulting solution was poured onto crushed ice , and the product that separated out was filtered off, washed with solution of sodium hydrogen carbonate followed by water and then dried. The products were recrystallized from a proper solvent.
Saccharinyl (N’- benzoyl methyl) acetamide(7)
A solution of oxazolonederivative 3 (10 m mole) in dry benzene was treated with anhydrous Alll3(30m mole) with continuously stirring on water bath for 3h.
The reaction mixture was decomposed with ice- cold hydrochloric acid. Then the ethereal layer was separated and dried over anhydrous Na2SO4.
The excess solvent was evaporated then the separated compound was recrystallized from benzene to give (7) : buff (60%) , mp 115-117 , IR (KBr, cm-1), 1700 (CO ketone), 1680 (CO imide) , 3340 (NH), 1330 and 1140 (SO2) MF C17H14O5N2S.EI-MS: m/z 358 (M+)
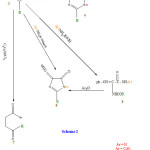
Oxo -1, 2 ,4 – triazino (4,3-b) (1,2) benzoisothiazole(9)
A mixture of compound 8 ( 10 m mole) in redistilled acetic anhydride 20ml was refluxed for 1h. the reaction mixture was cooled and poured onto ice – cold water. The solid product separated was filtered and recrystallized from ethanol to afford compound (9), yellowish brown (65%) , mp 145-147, IR (KBr, cm-1) : 1710 (CO) , 3432 (OH) , 3100 (NH) 1634 (C=N) 1363 and 1133 (SO2) , HNMR (DMSo-d6) S: 2.5 (s, 2H, cH2), 4.4 (s,1H, NH) , 5.7 (s. 1H, OH of lactam- lactim dynamic equilibrium) and 7.8-8.1 (m, 4H , ArH), MF C9H7N3O3S, EI-ms, m/z 251 (M+).
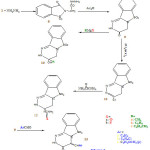
General procedure for the preparation of 6 – alkyl–6-hydroxyl– 1,2,4- triazino (4,3-b) (1,2) benzisothiazole (10a-c).
To Mg metal (10m mole) in dry ether (40ml) an alkyl halide namely, ethyl iodide , methyl iodide or benzyl chloride (30m mole) in dry ether (20ml) was added dropwisely. The reaction mixture was refluxed and compound a (10m mole) in dry ether (40ml) added portion wise within 1h.
The reaction mixture was further refluxed for 3h, left over night and then decomposed with dil cold HCl. The ethereal layer was / washed with NaHCO3 solution then water and dried over analydrous Na2 SO4 and evaporated to give compound 10a-c which was recrystallized form benzene (10a) : ball yellow (6690) , mp (195-197, IR( KB6, cm-1)) 3420 -3390 (OH and NH) , 1610 ( C=N) , 1310 and 1130 (SO2) , MF C10H11N3O3S, EI –MS: m/z 3(M+).
(10b) : yellowish (70%) , mp 172-174 ; IR ( KBr, cm-1)
: 3410 – 3380 ( OH and NH), 1600 (C=N), 1310 and 1110 (SO2) , ‘HNMR(DMSO-d6) S: 1.7 (t,3H,CH3) , 2.5 (q,2H,CH2) , 4(S,IH,OH), 4.5 (S,2H, CH2, N-CH4) , 7.8 (m, 4H , A,H) , 9.1 (S, 1H, NH). MFC11H13N3O3S. EI – HS: m/z 267 (M+).
(10c) yellow (60%) ,mp 205-207 , IR (KBr, cm-1) 3400-3370 (OH, and NH) , 1630 (C=N) 1320 and 1120 (SO2) . MF (C16H13N3O3S).
EI – MS : m/z 329 (M+)
6- chloro -1,2,4- triazino – (4,3-b) (1,2) benzisolhiazole(11)
A mixture of compound 3(10m mole) , phosphorous oxychloride ( 20m mole) and PCl5 (1qm) was refluxed on a steam bath for 3h.
Then poured slowly into ice – cold water. The solid that separated was washed several times with water, dried and recrystallized from benzene to give compound (11); brown (70%) ,mp 105-107. IR(KBr, cm-1), 1630 (C=N), 1330 and 1120 (SO2) and no absorption for CO and NH.
MF C9H6N3O2S C1. EI – MS’ : m/z 255 (M+) and 257 (M++2).
Formation of compounds ( 12 a and b)
A mixture of compound 11 ( 10m mole) was refluxed with urea and / or thio- urea ( 10 m mole ) in ( 40ml) sodium ethoxide for 3h, then cooled and poured into water. The solid that separated was dried and recrystallized from benzene – ethanol (1 :1) to give 12a and for 12b.
Compound (12a) : yellow (72%) , mp 72 -74 . IR (KBr, cm-1) 3350 -3320 (NH), 1610 (C=N) , 1310 and 1120 (SO2). MF C10H9N5O3S2 EI- MS: m/2 279 (M+ ).
Compound (12b) : yellowish (65%) , mp 235- 237. IR ( KBr, cm-1) 3370 -3330 (NH), 1630 (C=N), 1240 (C=S) 1310 and 1120 (SO2). ‘HNMR(DMSO-d6) :
4.2 (S2H, CH2, N-CH2) , 5.9 (2S, 2H, NH2) 7.5 (m, 4H , ArH), 8.2 (S, IH, NH).
MF C10H9N5O2 S2. EI – MS : m/z 295(M+).
6- OXO -5- arylidene – 1,2,4-triazino – (4,3-b) (1,2)- benzisothiazole (13a-c).
A mixture of compound 9 (10m mole) and aromatic aldehyde (10m mole) namely Benz aldehyde , p- chloro- Benz aldehyde and anisaldehyde in (40 ml).
Acetic anhydride – acetic acid (1: 1) was refluxed for . 3h. after concentrated, the solid that separated was cooled and recrystallized from benzene – ethanol (1: 1) to give (13a –c).
Compound 13a yellow (75%) ,mp 105-107.
IR (KBr, cm-1) 3400 -3360 (br. NH, OH), 1720 (CO), 1560 , 1480 (C=N, C=C), 1350 and 1150 (SO2). MF C16H11N3O3S. EI –MS : m/z 325 (M+).
Compound 13b: yellow (75%) ,mp 200 -202. IR (KBr, cm-1) 3420-3380(br. OH and NH), 1700 (CO), 1580, 1460 ( C=N, C=C) , 1330 and 1120 (SO2), MF C16H10N3O3SC1. EI- MS: m/x 359 (M+) AND 361 ( 2).
Compound 13c : ball yellow (65%) , mp 188-190. IR (KBR, cm-1) 3450- 3400 (br: OH and NH), 1720 (CO) , 1610, 1450 (C=N, C=C), 1300 and 1110 (SO2). MF C17H10N3O4S. EI- MS: m/z 355(M+).
 |
Scheme 1 Click here to View scheme |
 |
Scheme 2 Click here to View scheme |
 |
Scheme 3 Click here to View scheme |
Antimicrobial
The newly synthesized compounds (3,4a-b, 5a-b, 5d, 5g, 6c, 7, 9, 10a, 11, 12b, and 13a)were tested for their in vitro growth inhibitory activity against a panel of standard strains of the Institute of fermentation of Osaka (IFO) namely; theGram-positive bacteria (Staphylococcus aureus IFO3060 and Bacillus subtilis IFO 3007), the Gram negative bacteria (Escherichia coli IFO 3301 and Proteus vulgaris IFO 3851.
The primary screening was carried out using the agar disc-diffusion method using Müller-Hinton agar medium.24-28
Results and Discussion
Chemistry
In the present investigation, saccharinyl acetic acid chloride 9reacted with glycine to give N- carboxymethylsaccharinylacetamide(2), which reacted with benzaldehyde in the presence of acetic anhydride and sodium acetate to give 4- benzylidene-2-saccharinyl-methyl-1,3-oxzazole-5-one (3). Treatment of (3) with hydrazine and/or phenylhydrazine in refluxing ethanol afforded 5-benzylidene-3-sacchrinyl-methyl-1, 2, 4 triazine-6-one (4a) and/or 5-benzylidene-3-sacchrinyl-methyl-2-N-phenyl-1, 2, 4- triazine-6-one (4b). Furthermore when compound(3) reacted with aromatic amines (aniline, p-toludine) and aminobenzoic acids (m, and p-aminobezoic acid) in refluxing ethanol it gave cinnamimide derivatives (5 a-d). But when the same reaction was carried out in n-butanol it gave imidazolone derivatives (6a-d) which were also obtained by refluxing compounds (5a-d) in acetic anhydride. Moreover compound (3) undergoes acid catalyses ring opening reaction with dry benzene in presence of anhydrous AlCl3 (Fridel – Crafts reaction) to give saccharinyl – (N-benzoyl-methyl) acetamide (7), wherethe reaction product obtained via elimination of arylidene group23, 24 On the other hand, when saccharinyl acetic acid hydrazide (8) 9 was refluxed in acetic anhydride it cyclized to 6-oxo-1,2,4-triazino-[4,3-b][1,2] benzisothiazole (9).
Also the behavior of compound (9) towards carbon nucleophiles (Grignard reagent) was investigated. Thus when compound (9) submitted to react with ethyl magnesium iodide, methyl magnesium chloride then refluxed in dry benzene, it afforded 6-alkyl-6-hydroxy-1,2,4-triazino-[4,3-b][1,2]benzisothiazole (10). Treatment of compound(9) with mixture of POCl3/PCl5 afforded 6-Chloro-1,2,4-triazino[4,3-b][1,2]benzisothiazole (11) which reacted with urea and/or thiourea to give compound (12 a and b) respectively.
Also when compound (9) was allowed to condense with aromatic aldehyde namely bezaldehyde, anisaldehyde, and p-chlorobezaldehde in the presence of pyridine in a mixture of acetic anhydride and acetic acid (1:1) it afforded 5-arylidene-6-oxo-1,2,4-triazino-[4,3-b] benzisothiazole (13a-c).
Antimicrobial Activity
The antimicrobial activity (in vitro) of some synthesized compounds was determined against some bacteria and fungi using tetracycline and amphotericin B as standard antimicrobial agents by using the agar diffusion method28, 29
The obtained zones of inhibition were presented in table 1, which indicated that most of the synthesized derivatives have moderate to good antimicrobial activities.
Table 1: Antimicrobial activity (in vitro) of some synthesized compounds
|
Compd no. |
Zone of inhibition mm/ mg of sample |
|||
|
Escherickcacoli |
Staphylococcus aureus |
Candida albicans |
Aspergillusflavus |
|
|
3 |
0 |
0 |
17 |
15 |
|
4a |
0 |
0 |
15 |
0 |
|
4b |
18 |
12 |
15 |
18 |
|
5a |
0 |
0 |
15 |
0 |
|
5b |
0 |
0 |
16 |
0 |
|
5g |
0 |
0 |
16 |
0 |
|
5d |
12 |
14 |
0 |
0 |
|
6c |
16 |
12 |
15 |
0 |
|
7 |
15 |
10 |
15 |
0 |
|
9 |
0 |
0 |
10 |
0 |
|
10a |
19 |
0 |
10 |
0 |
|
11 |
0 |
0 |
15 |
0 |
|
12b |
0 |
0 |
0 |
0 |
|
13a |
18 |
0 |
0 |
0 |
|
Tetracycline |
30 |
28 |
0 |
0 |
|
Amphotericin |
0 |
0 |
16 |
20 |
References
- Magid Abou-Gharbia, John A.Moyer, UshaPatel, Micheal Webb, Guy Schiehser, Terrance Andree and J. Thomas Haskins, J. Med Chem.,1989,32, 1024
- Richard Poul Dunlap, Neil Warren, Albert Mura and Dennis John Hlasta, (Sterling Drug, Inc.), PCT Int. Appl.,1990,549,15.US Appl.1989,347, 125,111pp.
- Soon Kyoung Kwon and Myoung Suk, Arch. Pharmacal Res.1992,15, 251-255
CrossRef - Donald Josef Dumas, EP 165003 A2US 85-72 6452, 1985, Appl, 111pp.
- M. M. H. Arrief, M.S Amine and A.M.F Eissa, Egypt. J.Chem.,1999,42(6), 563-571
- “M.S. Amine and M.M.H.Arief, Indian J.Chem.,1998,37,135-138
- M.M.H Arief, S.G Donia, M.H.Azab and M.G Zinhong, Egypt,J.Chem,1998,41(1-6), 257-266
- M. M. H. Arief, Phosphorous, Sulfur, and Silicon,1997,127, 159-165.
CrossRef - M. M. H. Arief, Phosphorous, Sulfur, and Silicon,1996,114, 129-134.
CrossRef - A.A Failli, Us 4859671, 1989, Chem, Abst,1990,112,7717.
- D.J. Hlasta, R.C.Desai, C.Subramanyam, E.P.Lodge, R.P. Dunlap, N.W.Boaz, A.J. Mura, and L.H. Latimer, Eur.Pat.Appl. EP542372A1, 1993, Chem. Abst.1994,120(15), 191707,.
- W.C.Groutas, N. Houser – Archield, L.S.Chong, R.Venkataraman, J.B. Epp, H.Hunag and J.J. McCLenahan, J.Med.Chem.1993,36,3178-81, Chem. Abst.1993,119,225875
- N-Terzioglu, A.Gursoy, Eur.J.Med.Chem,2003,38,781
- G. Kolavi, V.Hegde, I.Khan, P.Gaded, Bioorg.Med.Chem.2006,14, 3069.
CrossRef - A.K. Gaded, C.S.Mohajanshetti, S.NimalKar, Eur.J.Med.Chem,2000,35, 853
- M.Kidwai, P.Mothsra, Tetrahedron Lett.2006,47, 5029
CrossRef - C. S. Andotra, T.C. Langer, A. Kotha, J. Indian Chem. Soc.
- S. A. Siddiqui, Tetrahedron Lett,2005,61, 3539
CrossRef - H. C. Kan, Tetrahedron Lett.2007,63, 1644
CrossRef - D. Luca, L.Curr, J. Med. Chem,2006, 13, 1
- Adel A.H. Abdel-Rahman, shafey G. Donia, A.A.F. Wasfy, A.A. Aly and Amaal Y. El-Gazzar, Der Pharma Chemica,2013, 5(1),196-204
- R. Cruickshank, J.P. Dugui, B.P. Marion, R.H.A Swain, Medicinal Microbiology 12thEdn. Churchill London,(1975),196.
- Murray PR, Baron EJ, Pfaller MA, TenoverFC, Yolken RH. Am. Soc. Micobiol.,Washington D.C. 1995
- Atlas RM. Handbook of MicrobiologicalMedia. London: CRC Press 2004,1226
- Villanova PA. National Committee for Clinical Laboratory Standards (NCCLS)Approved standard document M-7A,1985
- Sandven P, Lassen. Importance of Selective Media for Recovery of Yeasts from Clinical Specimens. J. Journal of clinical microbiology,1999, 37(11),3731–3732
- Magda H. Abdellattif, Mai. M. Helmy, Hany A. Eldeab, IJAPBC – 2014,3(4),
- Rishiramparajuli, janmajoybanerjee, and hemantakhanal.oriental journal of chemistry,2015,31,(4).2099-2106
- Reza moradivalikboni, zabialahheidarnezhad, fatemehheidarnezhad, yuldashboyhozhiboevandrahmanrahmanov,oriental journal of chemistry,2014, 30(1),391-394.
- ChalakAzimi, Farhad Sepehraddin and Helal Tahazadeh, ORIENTAL journal of chemistry,2013,29(4),1443-1448.

This work is licensed under a Creative Commons Attribution 4.0 International License.









