Synthesis of Ionic Imprinted Polymer Particles for Selective Membrane Transport ofFe(III) using Polyeugenol as the Functional Polymer
Muhammad Cholid Djunaidi1,2,*, Jumina2, Dwi Siswanta2 and Mathias Ulbricht3
1Department of Chemistry, Faculty of Science and Mathematics, Diponegoro Univ. of Indonesia 2Department of Chemistry, Faculty of Mathematics and Natural Sciences, GadjahMada Univ.of Indonesia 3Lehrstuhl für Technische Chemie II, Fakultät für Chemie, Universität Duisburg-Essen, Essen, Germany Corresponding Author Email: choliddjunaidi@live.undip.ac.id
DOI : http://dx.doi.org/10.13005/ojc/320107
Article Received on :
Article Accepted on :
Article Published : 03 Mar 2016
The preparation of Ionic Imprinted Polymer (IIP) particles for selective membrane transport of Fe (III) had been done using polyeugenol as functional polymer and PVA (polyvinyl alcohol) (Mr 125,000) solution in 1-Methyl-2-pyrrolidone (NMP) solvent as membrane base. The membrane was then cut and Fe(III) was removed by acid to produce IIP particles membrane. Analysis of the membrane and its constituent was done by IR, SEM and also TOC analysis. Experimental results showed the transport of Fe(III) was faster with the decrease of membrane thickness and the higher concentration of template. However, the transport of Fe(III) was slower for higher concentration of PVA (Polyvinyl Alcohol) in the membrane. The selectivity of all IIP particles membrane was confirmed as they were all unable to transport Cr (III), while NIP (Non-imprinted Polymer) membrane was able transport Cr (III).
KEYWORDS:IIP particles membrane; selective transport; crosslinking; polyeugenol
Download this article as:| Copy the following to cite this article: Djunaidi M. C, Jumina, Siswanta D, Ulbricht M. Synthesis of Ionic Imprinted Polymer Particles for Selective Membrane Transport ofFe(III) using Polyeugenol as the Functional Polymer. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Djunaidi M. C, Jumina, Siswanta D, Ulbricht M. Synthesis of Ionic Imprinted Polymer Particles for Selective Membrane Transport ofFe(III) using Polyeugenol as the Functional Polymer. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14529 |
Introduction
Considerable attention has been given to molecularly imprinted polymer (MIP) technology today, which leads to the increase of its application in many different fields of research ranging from filtration, chromatography, sensor [1], racemic mixture purification, as well as controlled catalytic complex chemical reaction [2].
Today, heavy metal imprinted adsorbents used for the separation of selective heavy metal have attracted more attention, one of which is for the separation of Fe (III) [3-4]. The development of inexpensive adsorbent with high adsorption capacity had been the main goal of many studies. One interesting study is the use of biomaterials as adsorbent for heavy metal waste [5,6]. Biomaterials are very important because it is cheap and biodegradable, as well as biocompatible. They can be prepared from a number of different agricultural waste, such as corn husk, bagase, rice husk, lignin, microbial biomass, chitosan [7,8],husk of green gram (phaseolusaureus) seed [9] daneugenol. Eugenol is one of native Indonesian natural products and has many purposes, including for the separation of metal ions. Some examples are the conversion of eugenol into polyeugeniloxyacetate was used for the separation of heavy metal mixtures by solvent extraction method [10]; and its conversion into eugenoxy acetate for the separation of Cr(III) using Bulk Liquid Membrane (BLM) method [10]. Eugenol polymer, polyeugenol, has been used as a BLM carrier with the order of Cr(III) Fe(III)>Ni(II) Zn>Cd (hard medium>soft) [12]. Djunaidi et al (2015) used polyeugenol as a functional polymer of IIP (Ionic Imprinted Polymer) method for selective adsorption of Fe(III)[13, 14].
In the last decade, imprinting techniques is used for the build up of molecularly imprinted membranes (MIMs) that can selectively recognize target molecule in a solution during a simple static adsorption as well as permeation through a membrane device [15].Djunaidi et al (2015) alsosynthesized IIP Fe membrane via simultaneous formation of IIP stucture and morphology (in situ) by used polyeugenol as a functional polymer [16].
The term membrane is used to describe an interface between two adjacent phases which acts as a selective barrier and control the transport between the two compartments [17-18] .A MIM is a membrane either composed of a MIP or containing a MIP [19]. In the present study, the synthesis of MIM (IIM) from IIP particles had been studied. Membrane preparation was preceded by the synthesis of IIP Fe(III) using polyeugenol as the functional polymer and PEGDE as the crosslinker. The IIP was then used to synthesize the membrane using PVA as the membrane base. PVA membranes usually display high stability in strongly acidic and alkaline environments [20].
Experiment Section
Instruments
The instruments used in this study are pH-meter, magnetic stirrer, separatory funnel, glasswares and plastic wares, analytical balance (Mettler Toledo AB54-S), three-necked flask, casting machine (Erichsen), FTIR Spectrophotometer (Jasco Miracle ATR), XRD (Shimadzu XRD-8000), AAS (Atomic Absorption Spectrophotometry (Shimadzu 8201PC), UV visible 50 Probe, TOC- analyser (SHIMADZU),SEM EDX (JSM 6380 LA), a set ofdifussion transport cell..
Materials
The materials used in this study are Eugenol, BF3O(C2H5)2and PEGDE (Polyethyleneglycoldiglycidylether) that were purchased from SIGMA-Aldricht, 1 Methyl 2 pyrrolidone and Fe(III) nitrat nonahydrate were purchased from Fluka, NaOH, HCl, polyvinylalcohol (PVA, Aldricht Mr. 125000), aquademineralized (Milli-Q).
Synthesis of Polymers
Polyeugenol.Eugenol (5.8 g) was put in a 3-necked flask, then 0.25 mL of boron trifluoride diethyl ether, BF3O(C2H5)2 was added as catalyst. The addition was done 4 times every hour while stirring with magnetic stirrer at room temperature. The occurrence of a reaction can be characterized by the color change of the solution into red. After the last addition of the catalyst, the polymerization was allowed to continue up to 12-16 hours, after which 1 mL of methanol was added to stop the reaction. The gel produced was dissolved in chloroform and put into a separating funnel and then washed repeatedly with distilled water until neutral. The organic layer was transferred into a 50 mL erlenmeyer flask and added with anhydrous Na2SO4. The liquid was separated by decantation. Afterwards, the solvent was evaporated in rotary evaporator at 40 °C. The residue obtained was further dried in the desiccator, and was subsequently weighed and characterized using FT-IR.
Synthesis of IIP-Fe(III) Particles Membrane
Polyeugenol (0.5 g) was stirred with (Fe (III) solution with different concentrations for 24 hours. The product was filtered with a filter paper and subsequently air dried at room temperature. 0.3 g of Polyeugenol-Fe(III) produced from this process was then crosslinked using PEGDE as the crosslinkerwith a mole ratio of 1:1 by heating for 15 minutes at 80-90 °Cwith 20 mL 1M NaOH as catalyst. The product was then neutralized and dried at 115°C in an oven for 6 hours. The polymer produced was further treated with acid for 24 hours to release the Fe(III) ions and form the final product of IIP-Fe(III) polymer.
The preparation of IIP particles membrane. 0.25 g of IIP Fe was added with 0.5 g PVA (weight ratio of IIP:PVA was 1:2) and dissolved in 7 mL NMP and then heated at 100-100 oC for 4 hours. Next, casting was carried out at 25m/s on a glass layer. The casting layer was then dried in an oven at 80 °C overnight. The membrane produced was then coagulated in 1M NaOH solution overnight. The membrane sheet obtained was cut and subsequently washed with demineralized water and the template was then leached using 0.1M HCl overnight and mounted onto the ring of the diffusion cell.
Synthesis of Non-Imprinting Polymer (NIP) membrane
NIP was synthesized using similar method as the synthesis of MIP, excluding the step of Fe(III) binding to the polymer.
Transport of Fe(III) ion through the membrane
Thetransport of Fe(III) ion through the membrane was carried out in a difussion cell, using 50 mL Fe(III) solution at 325 mg/L in the feed phase (pH 3).
Performance test for the Fe(III) IIP particles membrane
Optimization of Fe(III) IIP membrane performance was done for different membrane thickness, templates and PVA weight.
Selectivity test for IIP particles membrane
The selectivity of IIP membrane was compared to that of NIP membrane and the test was carried out using 50 mg/L Cr(III) solution(pH 3) in separate systems.
Total Organic Carbon (TOC) measurement
Diffusion measurements were performed to determine the rate of diffusion through the corresponding membranes (effective diffusion coefficient) and the solute fractionation under these conditions. This measurement was carried out using a diffusion cell composed of two half-cells. The membrane was fixed between these half-cells with the membrane side. The two half cells were filled at the same time to the same height level with receiving solution (water; 50 ml) and feed solution (10 g/l equimass mixture of dextrans with average molecular weight of 15000 until 20000 (dextran 15) in water ; 50 ml) and stirred at the same stirring rate. The diffusion of dextran through the membrane was monitored by measuring the concentrations using TOC in both half-cells at 24 hours.
Contact Angle measurement.
Contact Angle was measured by microscope instrument and then counted manually.
Analysis of Fe(III)
The analysis of Fe(III) was done by UV-visible spectrophometer using KSCN reagent at 458 nm and Atomic Absorption Spectrophotometry (AAS).
Analysis of Cr(III) with UV–Visible spectrophotometer
The analysis of Cr(III) was done by UV-Visible spectroscopy at 420 nm.
Results and Discussions
Synthesis of IIP Fe Particles membrane
The results of synthesis of IIP Fe particles membranewere then characterized using FTIR.
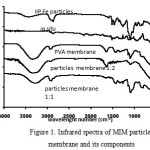 |
Figure 1: Infrared spectra of MIM particles membrane and its components Click here to View figure |
From Figure 1 it can be seen that the spectra for particles membrane can be seen as a combination of MIP particles and PVA membane spectra. The spectra of the particles membrane with weight ratio of 1:2 is dominated by PVA absorption characteristics, while the one with weight ratio of 1:1 is dominated by the characteristics of poly-PEGDE adsorbent particles, especially for wave number of 1100 cm-1, which is the typical absorption band for C-O of glycidyl ether. The spectra are different from that of in situ membrane as there are spectral shift due to new bonds being formed in the latter[15].
The transport of Fe(III) in the IIP particles membrane
The study of Fe(III) transport was carried out through a series of experiments using 25 mg/L Fe(III) solution at pH 3 and 0.1 HCl solution as the stripping phase. The result of Fe (III) transport from the feed phase to the stripping phase can be seen in Figure 2.
It can be seen in Figure 2 that the concentration of Fe(III) in the feed phase decreased with time as Fe(III) was transported to the stripping phase so that the concentration of Fe(III) in the stripping phase increased with time.
Membrane thickness variation
The effects of membrane thickness on membrane selectivity and flux had been studied by other researchers. Binning et al. had observed that the flux of n-heptane and isooctane mixture (50/50 vol%) through a plastic membrane is inversely proportional with membrane thickness and selectivity and was not affected by membrane thickness for the range of 20–50 µm[20].
Therefore, to optimize the performence of the membrane, thiner membrane (300 µm) needs to be synthesized. The results of Fe(III) transport using this membrane can be seen in Figure 2.
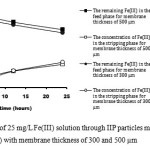 |
Figure 2: The transport of 25 mg/L Fe(III) solution through IIP particles membrane (weight ratio of IIP: PVA equals to 2:1) with membrane thickness of 300 and 500 µm Click here to View figure |
From Figure 2, it can be seen that for the membrane with membrane thickness of 300 µm, Fe(III) being transported to the stripping phase is higher than that of 500 µm membrane, which means that the amount of Fe(III) being trapped in the membrane is smaller. It indicates that the smaller thickness of membrane the higher % transport Fe(III) to stripping phase.
Variation of Concentration of Template
Template is one of the most important parts of IIP [13] and also IIP membrane in situ [16] as well IIP particles, as ilustrated in Figure 3.
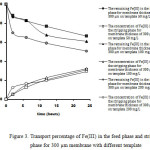 |
Figure 3: Transport percentage of Fe(III) in the feed phase and stripping phase for 300 µm membrane with different template |
It can be seen in Figure 3 that the transport percentage of Fe(III) from the feed phase increased with increasing template concentration for 300 µm membranes. Interestingly, the concentration of Fe(III) in the stripping phase decreased with increasing template concentration, which suggests that at higher template concentration, more imprinted caves were formed in the membrane which resulted in stronger interactions between the membrane and Fe(III) ions, so that the ions are more difficult to be released.This happenedproblaby because IIPparticles membrane works through retarded permeation mechanism [17-18].
Variation of membrane base (PVA) amount
To determine the effects of membrane base (PVA) amount on transport of Fe a series of Fe(III) transport experiments were carried out for IIP particles membrane synthesized with different PVA concentration. The weight ratio used for IIP polymer: PVA were 1:2 and 1:1, and the transport experiments results can be seen in Figure 4-5.
It can be seen from Figures 4-5 that the transport percentage of Fe(III) in the stripping phase increased and decreasing in feed phase with decreasing PVA concentration.
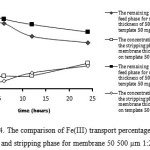 |
Figure 4: The comparison of Fe(III) transport percentage in the feed phase and stripping phase for membrane 50 500 µm 1:2 and 1:1. |
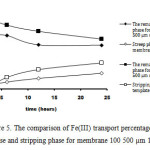 |
Figure 5: The comparison of Fe(III) transport percentage in the feed phase and stripping phase for membrane 100 500 µm 1:2 and 1:1 |
Scaning Electron Microscope (SEM)
The complete results form SEM analysis are shown in Figure 6.
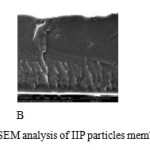 |
Figure 6: The results from SEM analysis of IIP particles membrane. (a) cross-section of the Click here to View figure |
membrane template 50 500 µm after transport of Fe (8000x magnification); (b) Front side of membrane template 50 500 µm after transport Fe (250x magnification) (c).Front side of membrane template 100 500 µm after transportof Fe (250x magnification)
As can be seen in Figure 6, the particles membrane appeared to be solid and unporous as well as symmetical. This is probably because the pores in IIP are blocked by the membrane base (PVA). This result was confirmed by the results obtained from size selective analysis using TOC analysis
Size selective analysis using TOC
Table 1: Size Selective Analysis Using Toc
|
Types of membrane (300 µm) |
TOC |
|
|
% dextran remaining in the feed phase |
% dextran in the water phase |
|
|
Membrane template 200 |
99.42 |
0.58 |
|
NIP |
93.76 |
0.98 |
As shown in Table 1, the membrane with 200 mg/L template could barely transport dextran 15, while NIP was able to transport almost 6% of dextran 15. This means that the particles membrane are nearly poreless, while the pores in NIP is larger that those in the membrane template 200particles.
Contact Angle
Table 2: Contact Angle Of Different Particles Membrane
|
Membrane Types |
Contact Angle |
| Membrane 1:2 thickness 300 µm | 45-50° |
| Membran 1:2 thickness 500 µm | 50-60° |
| Membran 1:1 thickness 500 µm | 50° |
As shown table 2 the thicker membrane the greater the contact angle, these happened problaby because the more content of the membrane.These contact angle value were similar to the contact angle of the IIP membrane in situ [16].
Membrane selectivity
The selectivity of the membrane was determined using 25 mg/L of Cr(III) solution. Cr(III) was selected because it has the same charge as Fe(III) and thus is expected to have similar charge density as well as ionic radius to Fe(III) [4]. The result obtained from the selectivity study can be seen in Table 1.
Table 3: Transport percentage of Cr(III) for different particles membrane
|
Type of membrane (IIP particles with 300µm and weight ratio of 1:2) |
% tranport of Cr(III) |
| NIP | 12.97 |
| Membrane template 50 | 0 |
| Membrane template 100 | 0 |
| Membrane template 200 | 0 |
As shown in Table 1, none of the MIMs was able to transport Cr(III), while NIP was still still able to transport Cr(III) to a certain extent, indicating the selectivity of MIMs synthesized in our study
Conclusions
MIM Fe (IIM Fe) particles prepared from PVA as membrane base, IIP particles and polyeugenol as functional polymer and NMP as solvent can be used for the transport of Fe(III). A number of factors were found to influence the performance of the MIM particles being synthesized, such as membrane thickness, template ion concentration, and the weight of the membrane base (PVA) used in the synthesis. The flux of Fe(III) was found to increase with the decrease of membrane thickness and the weight of the membrane base. In addition, The MIM particles synthesized is also selective, as demonstrated by the selectivity test using Cr(III), in which Cr(III) could not be transported, while NIP was able to transport some of the Cr(III) ions
Acknowledgment
- The first author would like to express sincere gratitude to the Government of the Republic of Indonesia for the Sandwich Program and Competitive Grant.
- The first author would like to also express sincere gratitude to the members of the group of Prof. Mathias Ulbricht because this research was mostly done during his stay at the University Duisburg-Essen.
References
- Trotta, F., Biasizzo, M., Caldera, F.,. Membranes,2012, 2, 440-477.
CrossRef - Mahony, J.O., Nolan, K., Smyth, M.R., Mizaikoff, B., Anal. Chim. Acta.2005, 534, 31-39.
CrossRef - Chang, X, Jiang, N, Zheng, H, He, Q, Hu, Z, Zhai, Y, Chui, Y., Talanta,2007, 7,38-43.
CrossRef - Xie, F., Lu, G., Wu, F., Guo, G., Li, G. Chem. Eng.,2012, 183, 372-380
CrossRef - Sölener, M., Tunali, S., özcan A.S., özcan, A., and Gedikbey, T.,Desalination, 2008, 223 (1-3), 308-322.
- Guibal, E., Sep. Purif. Technol., 2004,38 (1), 43–74.
CrossRef - Copello, G.J., Varela, F., Vivot, M., and Diaz, L.E., Bioresour. Technol., 2008,99 (14), 6538–6544.
CrossRef - Miretzky, P., and Cirelli, A.F., J. Hazard. Mater.,2009, 167 (1-3), 10–23
CrossRef - Jirekar, D.B.; Pathan, A.A.; Farooqui, M. Oriental Journal of Chemistry, 2014, 30 (3), 1263-1269
- Harimu, L, Matsjeh, S., Siswanta, D., Santoso, S.J., Indones J. Chem, 2009, 9(2), 261-266
- Djunaidi, M.C., Khabibi, Trisna, D.JSKA, 2010, 13, 2Djunaidi, M.C, Jumina, Siswanta, D., Ulbricht, M.,Indones. J. Chem.,2015, 15 (3)305 – 314.
CrossRef - Djunaidi, MC, Lusiana, RA, Wibowa, PJ, Siswanta, Dwi, Jumina, J. Alchemy. , 2007,6, 40-49.
- Djunaidi, M.C.,Jumina. Siswanta, D., Oriental Journal of Chemistry, 2015, 31(4), 2223-2229.
CrossRef - Algeiri, C., Drioli, E., Guzzo, L, Donato, L., Sensors,2014, 14, 13863-13912.
CrossRef - Ulbricht, M., Polymer,2006, 47, 2217-2262.
CrossRef - Djunaidi, M.C, Jumina, Siswanta, D., Ulbricht, M., Asian J. Chem., 2015, 27 (12), p. 4553-4562
CrossRef - Ulbricht, M, J. Chromatogr, B., 2004, 804, 113-125.
- Rhim, Ji-Won, Park, H.B., Lee, C.S., Jun, J.H, Kim, D.S., Lee, Y.M., , J. Membran Sci., 2004,238,143-151.
CrossRef - Villaluenga, J.P.G., Khayet, M., Godino, P., Seoane, B., Mengual, J.I..Separation and Purification Technology,2005,47, 80–87.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









