Synthesis, Characterization, and Antitumor Activity of New Organotin(IV) Methoxyethyldithiocarbamate Complexes
Normah Awang1, Nurul FarahanaKamaludin1, Ibrahim Baba3, Kok Meng Chan1, Nor Fadilah Rajab2 and Asmah Hamid2
1Environmental Health and Industrial Safety Program, Faculty of Health Sciences, UniversitiKebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur, Malaysia
2Biomedical Science Program, Faculty of Health Sciences, UniversitiKebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur, Malaysia
3School of Chemical Sciences and Food Technology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi Selangor, MalaysiaCorresponding Author Email: awang_normah@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320110
Article Received on :
Article Accepted on :
Article Published : 25 Jan 2016
Two new organotin(IV) dithiocarbamate complexes of the type R3SnL and R2SnL2 (L = methoxyethyldithiocarbamate and R = C6H5 or C4H9) were synthesized in good yields. The organotin complexes were suitably characterized by elemental analysis, FT-IR, 1H, and 13C NMR spectroscopies. These complexes were prepared in situ. Elemental analysis data (carbon, hydrogen, nitrogen, and sulfur) showed an agreement with the suggested formula structures. The infrared spectra of these complexes showed three important peaks for n(C=N), n(C=S), and n(Sn-S) in the region of 1450–1463, 992–994, and 324–326 cm−1 respectively. Data for 13C NMR spectroscopy showed an important peak in the region of dC197–201 ppm that corresponded to the NCS2 group. These complexes were evaluated for their in vitroantiproliferative activities against HL-60 cell lines. The results showed that both of these complexes had high cytotoxicities toward HL-60 cell lines with the IC50 values below 1 μM.
KEYWORDS:synthesis; organotin(IV); spectroscopy; dithiocarbamate; cytotoxicity
Download this article as:| Copy the following to cite this article: Awang N, Kamaludin N. F, Baba I, Chan K. M, Rajab N. F, Hamid A. Synthesis, Characterization, and Antitumor Activity of New Organotin(IV) Methoxyethyldithiocarbamate Complexes. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Awang N, Kamaludin N. F, Baba I, Chan K. M, Rajab N. F, Hamid A. Synthesis, Characterization, and Antitumor Activity of New Organotin(IV) Methoxyethyldithiocarbamate Complexes. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=13648 |
Introduction
Dithiocarbamates are sulfur- and nitrogen-containing ligands that display a rich and varied coordination chemistry, thus providing a wide range of transition and main group metal complexes (Hill et al. 1985). These ligands are versatile chelating agents with diverse applications in industry, agriculture, and medicine (Hulanicki 1967; Coucouvanis 1970). Dithiocarbamates deal with a great interest in inorganic synthesis as they have a number of applications. These ligands have attracted the attention among scientists because of their potential biological activities (Leka et al. 2006). Their metal complexes present striking structural features and have diversified applications such as high-pressure lubricants and accelerators used in vulcanization (Beer et al. 2001).
Some dithiocarbamates have also been found to be pharmacologically active, used for the treatment of alcoholism (Jacobsen 1950), and tested in clinical trials for various infections including HIV (Hersh et al. 1991; Kaplan et al. 1989; Lang et al. 1988) and cancers (Dufour et al. 1993; Francis et al. 1993; Verma et al. 1990). The antitumor effects of these dithiocarbamates can in part be attributed to their ability to complex tumor cellular copper, leading to binding to and inhibition of the proteasome and in turn initiating tumor cell-specific apoptosis (Buac et al. 2012). Besides, these dithiocarbamates have also been used in the treatment of bacterial and fungal infections (Menezes et al. 2004).
In view of the wide-range applications of organotin(IV) dithiocarbamate complexes, we report in this article the synthesis and characterization of dibutyltin(IV) and triphenyltin(IV) methoxyethyldithiocarbamate complexes and the cytotoxic study of these complexes to evaluate their potential as anticancer agents.
Experiment
Materials and Methods
All chemicals and solvents used in this experiment were purchased from Merck Company and used without purification due to their high purity. The melting points were determined in open capillary tubes using an electrothermal 9300 digital melting point apparatus. The percentage compositions of carbon, hydrogen, nitrogen, and sulfur were determined using an elemental analyzer, CHNS-O Model Fison EA1108. Solid state infrared spectra were recorded as potassium bromide discs using a Perkin-Elmer spectrophotometer GX. The 1H and 13C nuclear magnetic resonance spectra were recorded using a BRUKER FT-NMR 600 MHz Cryo-Probe spectrometer with DMSO-d6/CDCl3 as solvent.
Synthesis of Organotin(IV) Dithiocarbamate Complexes
Organotin(IV) dithiocarbamate complexes were prepared using a direct reaction between 5mmol of carbon disulfide and 5 mmol of ethanolic solution of methoxyethylamine. The reaction mixture was then stirred for 1 h at 277 K temperature and added dropwise to organotin(IV) (dibutyltin(IV) or triphenyltin(IV)) chloride (suitable amount) in 20 mL of ethanol. The precipitate formed was filtered and washed with cold ethanol and then dried in a desiccator. These complexes were then recrystallized from chloroform.
MTT Cytotoxicity Assay
The cytotoxicity of the synthesized compounds was screened using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazholium bromide (MTT) assay as described by Mosmann (1983). Briefly, the Jurkat, HL-60 (1 × 106 cells/mL) cells were treated with the synthesized compounds in a series of concentrations (0–10 uM) in a 96-well plate. Following 24 h of incubation, 20 μL of MTT (Sigma-Aldrich, USA) (5mg/mL in PBS solution) was added into each well while excluding ambient artificial lightand further incubated for 4 h. Then, 180 μL of supernatant was carefully removed from each well, and 180 μL DMSO (Fisher Scientific, UK) was added to dissolve the formazan crystals formed. After 15 to 20 min of incubation, the absorbance of each well was measured using an ELISA microplate reader (iMark) (Bio-Rad Laboratories, USA) at 570 nm. The graphs were plotted as a percentage of viable cells vs. compound concentrations. The IC50 values were determined based on the plotted graphs where the IC50 values represented the reduction of 50% of the cell population in the treated cells compared to the untreated cells. Doxorubicin hydrochloride was used as a positive control.
Results and Discussion
Synthesis
Two new organotin(IV) dithiocarbamate complexes were prepared using an insertion technique (Abdul Muthalib et al. 2011), which isa reaction involvingorganotin(IV) chloride, methoxyethylamine, and carbon disulfide at 277 K in an ethanol solvent to give stable complexes.The scheme for the reaction involved in the synthesis is shown in Figure 1. Both complexes exhibited as a white solid and wasstable in air and highly soluble in chloroform. Recrystallization process was done to the complexes using chloroform as a solvent. The resulting solution was slowly evaporated, and colorless crystals of the complexes were obtained. The elemental analysis showed that the experimental values were in agreement with the theoretical values based on their chemical formula (see Table 1).The percentage of tin in the complexes was determined using gravimetric analysis.
Reaction scheme of complex 1 (dibutyltin)
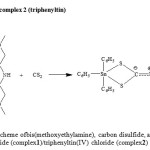 |
Figure 1: Reaction scheme ofbis(methoxyethylamine), carbon disulfide, and dibutyltin(IV) dichloride (complex1)/triphenyltin(IV) chloride (complex2) Click here to View figure |
Table 1: Physical and elemental analysis data for complexes1 and 2
|
Complex |
Yield (%) |
Melting point (°C) |
Elemental analysis % Found ( Calculated) |
||||
|
Carbon |
Hydrogen |
Nitrogen |
Sulfur |
Tin |
|||
|
1 |
76 |
68-69 |
41.76 (40.77) |
6.07 (7.14) |
4.91 (4.31) |
19.25 (19.75) |
27.38 (28.03) |
|
2 |
89 |
93-94 |
54.38 (53.76) |
4.38 (5.24) |
2.87 (2.51) |
12.13 (11.49) |
26.11 (27.00) |
Infrared Spectroscopy
The important infrared absorption bands of complexes1 and 2 are presented in Table 2. Based onthe spectral data, each complex exhibited a peak assigned as thioureide band, n(C=N) in the region of 1470–1500 cm−1 (Bonati&Ugo 1967; Sharma et al. 1996).The infrared spectra of thedithiocarbamatecomplexes showed very intense absorptions in the region of 1487–1470 cm−1, attributed to thioureidevibration (Honda et al. 1968).
Furthermore, another important peak observed inthese compounds wasn(Sn-C), and the peaks within the range544–559 cm−1 signified the presence the of Sn-C stretching bandsfor the compounds with phenyl or butyl moiety. The Sn–Sulfur coordination was supported by the presence of mediumabsorptions in the region of 386–425 cm−1, verifying thebonding of the tin metal with sulfur atom of the methoxyethyldithiocarbamateligand (Shahzadi et al. 2006;Santacruz-Juarez et al. 2008).
Table 2: Infrared spectra data of the organotin(IV) methoxyethyldithiocarbamate complexes
| Complex |
Wavenumber (cm−1) |
||||
|
n(C-N) |
n(C=S) |
n(C-H) |
n(Sn-C) |
n(Sn-S) |
|
|
1 |
1487 |
992 |
2921 |
544 |
386 |
|
2 |
1470 |
994 |
2935 |
559 |
425 |
NMR Spectroscopy
1H NMR spectra for these complexes were recorded in CDCl3 solution, and tetramethylsilanewas used as an internal standard at room temperature. The proton of methoxy group for both complexes exhibited a sharp singlet signal at dH 3.35 ppm (see Table 3). The protons of ethylene groups, N-CH2 and –CH2 attached to the nitrogen atom exhibited a triplet signal, respectively, at dH3.70 and dH4.13 ppm for dibutyltin(IV) complex and dH 3.72 and dH4.13 ppm for triphenyltin(IV) complex.
In the case of the dibutyltin(IV) compound, i.e., complex 1, a triplet signal was observed for the methyl protons at dH0.94 ppm, and three sets of broad signals for the methylene protons were also observed in the ranges ofdH1.41–1.47, 1.88–1.93, and 2.04–2.07 ppm. The attachment of the butyl group to the electropositive Sn atom via carbon nuclei would cause a shielding effect, experienced through the carbon chain (Gomez-Ortiz et al. 2002). A complex multiplet was found atdH7.27–7.74 ppm in triphenyltin(IV) complex due to the aromatic protons of the phenyl group directly attached to Sn atom (Khan et al. 2013).
Table 3:1H NMR data of the organotin(IV) methoxyethyldithiocarbamate complexes
| Complex |
1H NMR (ppm) |
|||
|
Sn-R (R=C4H9, C6H5) |
N-R’ (R’=CH2) | N-R’-R” (R”=CH2) | O-CH3 | |
|
1 |
–CH2: 2.06 (2H) 1.91 (2H) 1.45 (2H) –CH3: 0.94 (3H) |
3.70 (2H) |
4.13 (2H) |
3.35 (3H) |
|
2 |
CHaromatic: 7.40 -7.74 (5H) |
3.72 (2H) |
4.13 (2H) |
3.35 (3H) |
The important data of 13C NMR of these complexes are depicted in Table 4. The 13C NMR spectra of complexes1 and 2 exhibited signals for the O-CH3 carbon at the same chemical shift (dC 59.01 ppm). The chemical shift of the butyl carbon atoms attached to the tin atom in complex1 appeared atdC 13.87–34.26 ppm. The signal at the region dC 128.55–142.36 ppm was assigned to the aromatic carbons attached to the tin atom of complex 2 (Khan et al. 2015).The assignment of 13C signal for the NCS2 group for complexes 1 and 2 appeared at dC 201.52 and dC 197.26 ppm, respectively, which indicated that the coordination between sulfur and tin atomswas performed (Van Gaal et al. 1979).
Table 4: 13C NMR data of organotin(IV) methoxyethyldithiocarbamate complexes
| Complex |
Chemical shift, d (ppm) |
||||
|
N13CS2 |
O-CH3 |
Sn-R (R=C4H9 or C6H5) |
-O-CH2– |
-N-CH2– |
|
|
1 |
201.52 |
59.01 |
13.87, 26.41, 28.55, 34.26 |
70.07 |
55.59 |
|
2 |
197.26 |
59.01 |
128.55, 129.16, 136.78, 142.36 |
70.01 |
57.05 |
In vitro Cytotoxic Activity
The efficiency of the synthesized dibutyl- and triphenyltin(IV) complexes as potentialantitumor agents were preliminarily tested in vitro against HL-60 cell lines.The results of the in vitro cytotoxic activity of complexes 1 and 2(see Table 5 and Figure 2) against HL-60 cell lines were compared using doxorubicin hydrochloride as a positive control. Previous studies carriedout by our group demonstrated that theorganotin(IV) dithiocarbamate complexes are potent to be developed as anticanceragents (Awang et al. 2010; Khan et al. 2014a; Khan et al. 2014b). The values are expressed asIC50, i.e., the concentration of compound (in μM) that inhibits a proliferation rate of the tumor cellsby 50% as compared to the untreated cellsas a control.
Table 5: IC50values of the organotin(IV) methoxyethyldithiocarbamatecompounds on HL-60 cell lines
|
Complex |
IC50 values (mM) |
|
1 |
0.40 |
|
2 |
0.35 |
Note: IC50 (mM) isthe concentration that shows 50% inhibition of the cell population. The IC50 of doxorubicin hydrochloride is 0.275 µM.
 |
Figure 2: Graphical representation of the cytotoxic activity of complexes 1 and2 and doxorubicinagainstHL-60 cell lines.The red line (—–) indicates the 50 % of viable cells population. Click here to View figure |
The results signified that the testedorganotins induced a concentration-dependent antiproliferative effect toward HL-60 cells upon treatment for 24 h. These two complexes exhibited highantiproliferative effects;thus, this finding was in agreement with the previous study by Awang et al. (2010). Compared to the value oftriphenyltin(IV) complex, thedibutyltin(IV) complex displayed a higher potency against the HL-60 cells, and the IC50 values were on the other hand much lower. Therefore, both of these complexes have the potential to be developed as antitumor agents due to their potent cytotoxiceffect at micromolar concentrations. As these results are preliminary, further mechanistic studieson the antitumor activities of these complexes are highly recommended.
Conclusion
The formation of the dibutyltin(IV) and triphenyltin(IV) methoxyethyldithiocarbamate complexes were confirmed via characterization analysis. The micro elemental composition in both complexes were in good percentages and in agreement with the suggested molecular formulas. The presence of the thioureide bands, n(C=N) and the Sn-S stretching frequencies in the complexes indicated the formation of the dithiocarbamate groups and the bonding between the Sn(IV) with the dithiocarbamate ligands, respectively. The formation of dithiocarbamates were further supported by the NCS2 peaks recorded via 13C NMR. The cytotoxicity assay of thesecomplexes showed a high cytotoxic activity on the HL-60 cell lines.Further in vitro and in vivostudies are recommended as the next research stages on these potential complexes.
Acknowledgement
This work was supported by the grant FRGS/2/2013/SKK10/UKM/02/1.We gratefully acknowledge the School of Chemical Science and Food Technology, UniversitiKebangsaan Malaysia for providing the essential laboratory facilities. We would also like to thank the laboratory assistants from the Faculty Science and Technology, Universiti Kebangsaan Malaysia for their technical support.
References
- Abdul Muthalib, A.F., Baba, I., Abdul Aziz, Y.F. &Samsudin, M.W.. Synthesis and characterization of diphenyltin(IV) dithiocarbamate compounds. The Malaysian Journal of Analytical Sciences,2011,15(1), 106-112.
- Beer, P.D., Berry, N., Drew, M.G.B., Fox, O.D., Padilla-Tosta, M.E., &Patell, S.. Self-assembled dithiocarbamate–copper(II) macrocycles for electrochemical anion recognition. Chem. Commun. 2001, 4, 199–200.
CrossRef - Bonati, F. &Ugo, R..Organotin(IV) N,N-disubstitutedDithiocarbamates. Journal of Organometallic Chemistry.1967, 10, (2):257-268.
CrossRef - Coucouvanis, D.. The chemistry of the dithioacid and 1,1-dithiolate complexes. Prog.Inorg. Chem. 1970,11, 233–371.
CrossRef - Dufour P, Lang JM, Giron C, Duclos B, Haehnel P, Jaeck, D, Jung JM &Oberling F. Sodium dithiocarbamate as adjuvant immunotherapy for high risk breast cancer: a randomizedstudy. Biotherapy.1993, 6, (1) :9-12.
CrossRef - P. Francis, M. Markman, T. Hakes, B. Reichman, S. Rubin, W. Jones, J.L. Lewis, J. Curtin, R.Barakat, &M. Phillips. Diethyldithiocarbamatechemoprotection of carboplatin-induced hematological toxicity.Journal of Cancer Research and Clinical Oncology. 1993,119,(6), 360-362.
CrossRef - Hill, J.O., Magee, R.J. &Liesegang, J.. Photoelectron spectroscopy of metal dithiocarbamate, xanthate and dithiophosphate complexes: A review comments on inorganic chemistry. A Journal of Critical Discussion of the Current Literature.1985, 5(1): 1-27.
- Hulanicki, A..Complexation reactions of dithiocarbamates.Talanta.1967, 14(12): 1371–1392.
CrossRef - Leka Z.B, Leovac V.M, Lukic S, Sabo T.J, Trifunovic S.R &Katalin M.S..Synthesisandphysico-chemical characterization of new dithiocarbamato ligand and its complexes with copper(II), nickel(II) and palladium(II). J. Therm Anal and Cal. 2006, 83.
- Lang, J. M., Touraine, J. L.,Trepo, C.,Choutet, P.,Kirstetter, M.,Falkenrodt, A.,Herviou, L.,Livrozet, J. M.,Retornaz, G.,&Touraine, F..Randomised, double-blind, placebo-controlled trial of ditiocarb sodium (‘Imuthiol’) in human immunodeficiency virus in-fection.Lancet 1988, 332: 702-706.
CrossRef - Mosmann, T.. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods 1983, 65: 55-63.
CrossRef - Naqeebullah Khan, Yang Farina, KokMengChan, Lo KongMun, Nor Fadilah Rajab &ThengChoonOoi.. Diorganotin(IV) derivatives of N-Methyl p-fluorobenzohydroxamic Acid; preparation, spectral characterization, X-ray Diffraction Studies and antitumor activity. Molecules.2013, 18: 8696-8711.
CrossRef - Naqeebullah Khan, Yang Farina, Lo Kong Mun, Nor Fadilah Rajab,&NormahAwang. a. Triorganotin(IV) complexes with o-substituted arylhydroxamates: Synthesis, spectroscopic characterization, X-ray structures and in vitro cytotoxic activities.Journal of Organometallic Chemistry.2014,763–764: 26–33.
- Naqeebullah Khan, Yang Farina, Lo Kong Mun, Nor Fadilah Rajab &NormahAwang. Syntheses, characterization, X-ray diffraction studies and in vitro antitumor activities of diorganotin(IV) derivatives of bis(p-substituted-N-methylbenzylaminedithiocarbamates). Polyhedron. 2015,85: 754-760.
CrossRef
- NormahAwang, Ibrahim Baba, NorSyaidatulAkmalMohdYousof,&NurulFarahanaKamaludin..Synthesis and characterization of Organotin(IV) N-Benzyl-N-Isopropyldithiocarbamate Compounds: Cytotoxic Assay on Human Hepatocarcinoma Cells (HepG2). American Journal of Applied Sciences.2010, 7(8): 1047-1052.
CrossRef - Pellerito, C., Nagy, L., Pellerito, L. &Szorcsik, A. Biological activity studies on organotin(IV)n+ complexes and parent compounds.Journal of Organometallic Chemistry.2006,691(8):1733-1747.
CrossRef - Santacruz-Juarez, E., Cruz-Huerta, J., Hernandez-Ahuactzi, I.F., Reyes-Martinez, R., Tlahuext,H., Morales-Rojas, H., &Hopfl. H.. 24- and 26-membered macrocyclicdiorganotin(IV) bis-dithiocarbamate complexes with N,N’-disubstituted 1,3- and 1,4-bis(aminomethyl)benzene and 1,1′-bis(aminomethyl)ferrocene as spacer groups.Inorg. Chem. 2008,47(21): 9804-9812.
CrossRef - Sharma, J., Singh, Y., Bohra, R., &Rai, A.K..Synthesis and spectral studies of diorganotin heterocyclic dithiocarbamate complexes: The crystal structure of (CH3)2Sn[(S2CNCH2CH2CH2CH2CH2)]2. Polyhedron.1996, 15(7): 1097-1102.
CrossRef - Shahzadi, S., Ali, S., Bhatti, M. H.,Fettouhi, M. &Athar, M..Chloro-diorganotin(IV) complexes of 4-methyl-1-piperidine carbodithioic acid: Synthesis, X-ray crystal structures,spectral properties and antimicrobial studies. Journal of Organometallic Chemistry. 2006,691(8): 1797-1802.
CrossRef - Van Gaal, H.L.M., Dieskeld, J.W., Pijpers, F.W. & Van Der Linden, J.G.M. 13C NMR spectra of dithiocarbamates.Chemical shifts, carbon-nitrogen stretching vibration frequencies, and π bonding in the NCS2 fragment. Inorganic Chemistry. 1979,18(11): 3251-3260.
CrossRef - Verma, S., Stewart, D.J., Maroun, J.A. & Nair, R.C..A randomized phase II study of cisplatin alone versus cisplatin plus disulfiram.American Journal of Clinical Oncology.1990,13:119-124.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









