Potentiometric studies on the binary and mixed ligand complexes in solution: Zn(II) - Amlodipine - Amino acids systems
Lamia A. Al-bedair1,2
1Department of Chemistry, College of Science, Princess Nourah Bint Abdul Rahman University
2Deanship of Scientific Research, Princess Nourah Bint Abdul Rahman University
Corresponding Author Email: laalbedair@pnu.edu.sa
DOI : http://dx.doi.org/10.13005/ojc/320169
Article Received on :
Article Accepted on :
Article Published : 26 Jan 2016
Binary and mixed ligand complexes of Zn(II) involving amlodipine drug (A) as a primary ligand and some amino acids (L) as a secondary ligands are investigated. The acidity constants of the ligands were determined and used for determining the stability constants of the complexes formed in 80% (v/v) ethanol-water media under the experimental conditions at 25 ºC , I = 0.10 M (NaNO3) by using potentiometric technique. The potentiometric data were analysed using the computer program HYPERQUAD. The concentration distribution of the complexes in solution was evaluated.
KEYWORDS:Amlodipine; Amino acids; Stability constant; potentiometric
Download this article as:| Copy the following to cite this article: Al-bedair L. A. Potentiometric studies on the binary and mixed ligand complexes in solution: Zn(II) - Amlodipine - Amino acids systems. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Al-bedair L. A. Potentiometric studies on the binary and mixed ligand complexes in solution: Zn(II) - Amlodipine - Amino acids systems. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=13710 |
Introduction
Recently there has been considerable interest in the study of binary, ternary and quaternary complexes by pH-metric method [1-3]. The metal ions in presence of organic and inorganic ligands, amino acids, peptides, proteins, vitamins and enzymes are playing a major role in the field of analytical Chemistry [4,5]. The mixed ligand complexes have been studied extensively because of their potential role in biological processes and can manifest themselves as enzyme-metal ion substrate complexes [6-8]. The amino acids and drugs have a significant biological and medicinal importance. In view of the growing interest in the ternary complexes, it is thought worthwhile to study the ternary complexes of number of amino acids and amlodipine with zinc (II). Amlodipine, 2-[(2-aminoethoxy)methyl] 4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5- pyridinedicarboxylic acid 3-ethyl 5-methyl ester, is a second generation 1,4-dihydropyridine derivative of the prototypical molecule nifedipine [9]. The importance of pyridinecarboxylic acids stems from their presence in many natural products (alkaloids, vitamins, coenzymes, etc.). They are also of great interest to medicinal chemists because of the wide variety of their physiological properties displayed by the natural and synthetic acids. It is well known that complexes of metal ions are among the prominent interactions in nature [10,11], and versatile binding site of protein, amlodipine seems to have the best chelating properties. In the present investigation, the stability constants of Zn(II) complexes with amlodipine and some amino acids were studied in detail by potentiometric titration method in 80% (v/v) ethanol-water at 25 ºC and I = 0.10 M NaNO3. The simultaneous reactions of the complexes has been established in the pH region studied. The concentration distribution of the complexes in solution was evaluated.
Experimental
Materials and Solutions
Amlodipine, amino acids and related compounds, glycine, β-phenylalanine, alanine, valine, methionine, proline, threonine, histidine .HCl, histamine .2HCl, imidazol, ornithine .HCl, lysine, penicillamine, cysteine and mercaptoethylamine were obtained from Sigma Chemical U.S.A. All these chemical are used as received without any further purification, their purities ranged from 99-100%. Zinc(II) nitrate was provided by BDH-Biochemicals Ltd Poole, England. Their solutions were prepared and their concentrations were determined by conventional chemical methods[12]. Sodium hydroxide was prepared by dissolving an Analar product in deionzed water to obtain a saturated solution. The required solutions were prepared by careful dilution in deionized water (CO2 –free) and then standardized with potassium hydrogen. All solutions were prepared with deionized water.
Apparatus
Potentiometric titrations were performed at (25°C±0.1°C) in a double-walled glass vessel using a Griffin pH J-300-010 G Digital pH meter. The temperature was controlled by circulating water through the jacket, from a constant temperature bath. The electrode system was calibrated in terms of hydrogen-ion concentrations instead of activities.
Procedure and Measuring Techniques
The acid dissociation constants of the ligands were determined potentiometrically by titrating the ligand (40cm3) solution (0.005M) of constant ionic strength 0.1M, (adjusted with NaNO3) . The stability constants of the binary complexes were determined by titrating 40cm3 of a solution mixture of Zn(II) (0.005M), the ligand (0.01M) and NaNO3 (0.1M). The conditions for measuring stability constants of the ternary complexes were the same as those adopted for the binary ones, however the solution mixture (40cm3) contained (0.005M) of Zn(II) ,(0.005) amlodipine (A) and (0.005M) of the other ligand. Furthermore, the stability constants of the ternary complexes were determined using potentiometric data obtained from mixtures of Zn(II), A and other ligands in a concentration ratio of 1:1:1 for amino acids. All titrations were performed in 80% (v/v) ethanol-water media and in a purified N2 atmosphere using aqueous 0.05M NaOH as titrant. For all the titrations, HNO3 solution was added, so that they were fully protonated at the beginning of the titrations.
Data Processing
The calculations were obtained from ca.100 data points in each titration using the computer program HYPERQUAD [13]. The stoichiometries and stability constants of the complexes formed were determined by trying various possible composition models. The results obtained are shown in Table1.The concentration distribution diagrams were obtained using the program HYSS [14] under the experimental condition used.
Results and Discussion
The acid dissociation constants of the ligands were determined at 25.0 ºC, I = 0.10 M (NaNO3),and in 80% (v/v) ethanol-water media under the same experimental conditions used to study the Zn-A and the corresponding ternary complexes. The values were given in Table1. A pKa value of amlodipine 8.7 means that is predominantly present in the ionized form at a physiologic pH.
Formation equilibria of binary Zn(II)-A complexes
Potentiometric titration curves of A in presence and absence of Zn(II) ion are shown in Fig. 1. In the metal complex curve, there is a significant lowering from that of the free amlodipine, indicating formation of metal complexes by release of protons. Equilibrium models have been tried to fit the experimental potentiometric data for the Zn- (amlodipine). The selected model with the best statistical fit was found to consist of Zn(A) (110) and Zn(A)H (111) complexes. The concentration distribution diagram of various species as a function of pH is depicted in Fig. 2. The concentration of the 1100 and 1200 species increase with increase of pH, attaining a maximum of 80% and 95% at pH ca 7.40 and 10.20 , respectively. The main species present under physiological condition is calculated to be 1100 and 11200 which may be able to pass through cellular membranes.
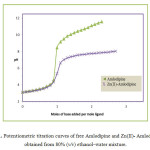 |
Figure 1: Potentiometric titration curves of free Amlodipine and Zn(II)- Amlodipine obtained from 80% (v/v) ethanol–water mixture. |
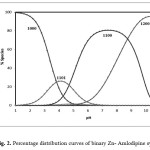 |
Figure 2: Percentage distribution curves of binary Zn- Amlodipine systems. |
Ternary complex formation equilibrium
Ternary complex formation may proceed either through a stepwise or a simultaneous mechanism depending on the chelating potential of amlodipine and other ligands. The formation constants of the 1:1 Zn(II) complexes with A and those of amino acids (Table1), are of the same order. Consequently, the ligation of amlodipine and amino acids will proceed simultaneously. The validity of this model was verified by comparing the experimental potentiometric data with the theoretically calculated (simulated) curve. Figure 3 presents such a comparison for the Zn-A-glycine system, taken as a representative one.
The general four component equilibrium can be written as follows (charges are omitted For simplicity):
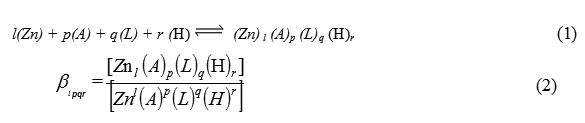
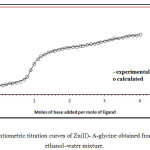 |
Figure 3: Potentiometric titration curves of Zn(II)- A-glycine obtained from 80% (v/v) ethanol–water mixture. |
The formation constants of amino acid complexes are higher than those of the corresponding monodentate imidazole complex. This indicates that amino acids bind through their amino and carboxylate groups. Phenylalanine forms a less stable complex than alanine. This may be due to the lower basicity of the amino group of phenylalanine compared to that of alanine. This will contribute to the decreased stability of the complex formed. The stability constant values of histidine and histamine are of the same magnitude and are considerably higher than those of the α- amino acids, indicating that both histidine and histamine would coordinate preferably through the amino and imidazole groups.
Concerning the secondary ligands (L): lysine, ornithine and histidine which can form protonated and deprotonated complexes, the acid dissociation constants of the protonated complexes are given by the following relation:
pKH = log β1111 − log β1110 (3)
The acid dissociation constants determined for the protonated ternary complex species amounted to 7.79 for ornithine and 7.73 for lysine, which compare favourably with the acid dissociation constant of the ω-amino group. This indicates that α-amino group is bound to Zn(II) ion in the ternary complexes while the ω-amino group remains free. The acid dissociation constant obtained for the protonated ternary complex with histidine is 5.78, being lower than that of the protonated amino group NH3+ (pKa = 9.53), but closer to that of the protonated imidazole group (pKa = 6.12), suggesting the proton in the protonated complex would be located mainly on the imidazole group.
The acid dissociation constants of ternary complexes with the sulphur ligands, penicillamine, cysteine and mercaptoethylamine are 6.13, 6.92 and 5.69 respectively. These values obtained in the present study are less than the previously reported microscopic acid dissociation constants [15] , revealing that the [–NH3]+ and –SH groups most likely take part in complex formation.
Evaluation of the concentration distribution of the various species as a function of pH provides a useful description of metal ion binding in biological system. In all species distribution curves the concentration of the formed complex increases with increasing pH , thus making the complex formation more favoured in the physiological pH rang. Protonated ternary complex species have been found to be most favoured at lower pH values.
The ∆ log K values are used to indicate the relative stability of the ternary formed through simultaneous mechanism, as compared to those of the corresponding binary complexes can be calculated using Eq. (4)
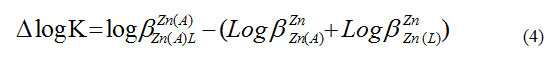
Table 1: Acidity constant of ligands and formation constant of the binary and ternary complexes of Zn(II) involving amlodipine and amino acids at 25◦C and 0.1 M ionic strength.
| System |
l |
p |
q |
ra |
log βb |
Δ log K
|
| Amlodipine |
0 0 1 1 1 |
1 1 1 2 1
|
0 0 0 0 0
|
1 1 0 0 1 |
8.26(0.01) 11.99(0.02) 6.23(0.05) 11.22(0.01) 13.01(0.02)
|
|
| Glycine |
0 0 1 1 1 |
1 1 0 0 1 |
0 0 1 2 1 |
1 2 0 0 0 |
9.32(0.01) 11.60(0.01) 5.07(0.06) 9.12(0.07) 12.44(0.03) |
1.14 |
| β-phenylalanine |
0 0 1 1 1 |
1 1 0 0 1 |
0 0 1 2 1 |
1 2 0 0 0 |
9.00(0.01) 11.04(0.01) 5.23(0.02) 9.51(0.01) 12.79(0.02) |
1.33 |
| Alanine |
0 0 1 1 1 |
1 1 0 0 1 |
0 0 1 2 1 |
1 2 0 0 0 |
9.33(0.01) 11.70(0.01) 4.59(0.03) 8.61(0.01) 13.01(0.05) |
2.19 |
| Valine |
0 0 1 1 1 |
1 1 0 0 1 |
0 0 1 2 1 |
1 2 0 0 0 |
9.39(0.01) 11.65(0.01) 4.34(0.02) 8.04(0.02) 12.44(0.01) |
1.87 |
Table 1 (Continued)
| System |
l |
p |
q |
ra |
log βb |
Δ log K
|
| Methionine |
0 0 1 1 1
|
1 1 0 0 1
|
0 0 1 2 1
|
1 2 0 0 0
|
9.12(0.01) 11.10(0.04) 4.47(0.04) 8.32(0.05) 12.03(0.01)
|
1.33 |
| Proline |
0 0 1 1 1
|
1 1 0 0 1
|
0 0 1 2 1
|
1 2 0 0 0
|
10.40(0.01) 12.00(0.02) 5.40(0.09) 10.79(0.06) 13.63(0.001) |
2.00 |
| Threonine |
0 0 1 1 1
|
1 1 0 0 1
|
0 0 1 2 1
|
1 2 0 0 0
|
9.06(0.01) 11.03(0.02) 5.11(0.02) 9.56(0.02) 13.98(0.01) |
2.64 |
| Histidine |
0 0 1 1 1 1 1 |
1 1 0 0 0 1 1 |
0 0 0 1 2 1 1 |
1 2 3 0 1 0 1 |
9.53(0.01) 15.81(0.03) 17.81(0.06 ) 6.71(0.03) 12.14(0.02) 15.67(0.01) 21.45(0.03) |
8.51 5.78 |
| Histamine |
0 0 1 1 1 1 |
1 1 0 0 0 1 |
0 0 1 2 1 1 |
1 2 0 0 1 0 |
9.85(0.01) 16.05(0.05) 5.26(0.06 ) 10.63(0.04) 13.23(0.01) 16.01(0.01) |
4.52 |
| Imidazol |
0 1 1 1 1 |
1 0 0 1 1 |
0 1 2 1 2 |
1 0 0 0 0 |
6.12(0.01) 3.98(0.02) 6.38(0.08) 10.32(0.01) 17.09(0.06) |
0.11 |
Table 1 (Continued)
| System |
l |
P |
q |
ra |
log βb |
Δ log K |
| Ornithine |
0 0 1 1 1 1 |
1 1 0 0 1 1 |
0 0 1 2 1 1 |
1 2 0 0 0 1 |
10.79(0.03) 10.49(0.01) 6. 39 (0.05) 11. 34 (0.06) 15.89(0.01) 23.68(0.02) |
3.27 7.79 |
| Lysine |
0 0 1 1 1 1 |
1 1 0 0 1 1 |
0 0 1 2 1 1 |
1 2 0 0 0 1 |
10.44(0.01) 19.66(0.01) 6.93(0.03) 12.08(0.05) 16.78(0.03) 24.51 (0.02) |
3.62 7.73 |
| Pencillamine |
0 0 1 1 1 1 |
1 1 0 0 1 1 |
0 0 1 2 1 1 |
1 2 0 0 0 1 |
10.10(0.01) 17.97(0.01) 9.80(0.01) 19.32(0.01) 16.89(0.01) 23.02(0.01)
|
0.86 6.13 |
| Cysteine |
0 0 1 1 1 1
|
1 1 0 0 1 1 |
0 0 1 2 1 1 |
1 2 0 0 0 1 |
9.77(0.03) 17.67(0.02) 8.45(0.07) 16.37(0.06) 14.96(0.04) 21.88(0.01) |
7.20 6.92 |
| Mercaptoethylamine |
0 0 1 1 1 1 |
1 1 0 0 1 1 |
0 0 1 2 1 1 |
1 2 0 0 0 1 |
10.03(0.04) 18.64(0.02) 9.61(0.04) 17.69(0.03) 21.44(0.02) 27.13(0.01) |
5.60 5.69 |
al, p and q are the stoichiometric coefficient corresponding to Zn(II), A, L and H+ respectively. bstandard deviations are given in parentheses. csum of square of residuals.
Acknowledgments
This research project was supported by a grant from the Deanship Of Scientific Research, Princess Nora Bint Abdul Rahman University.
References
- Marcus,Y. and Elizer, I., Coor. Chem. Review 1969, 4, 273.
CrossRef - M.T. Beck, The determination of complex equilibria, 1969, 172.
- Sigel, H., Metal ions in biological systems. Marcel Dekker Inc., New York, 2, 1 (1973); 5, 250 (1976), 6, 1(1976).
- Flaschka, H.A. and Bernard, A.J., Chelates in analytical Chemistry, 1967,. I, Marcel Dekker Inc., NewYork.
- Burger, K., Millor, I.T. and Allen, D.W., Coordination chemistry : Experimental methods. Butterworths Co.(Publishers) Ltd., London 1973.
- Vallee, B.L. and Coleman, J.E., Compr. Biochem. 1964, 12, 165.
- Malmstrom, B.G. and Rosenberg, A., Advan. Enzymol. 1959,21, 131.
- Mildvan, A.S. and Cohn, M., J. Biol. Chem. 1966,241, 1178.
- Beale Jr, J.M. and Block, J.H., Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed., Lippincott Williams and Wilkins, Philadelphia 2011., 625, 626
- Sigel (Ed.), H., Metal Ions in Biological Systems 1973,.2. Dekker, New York.
- Wood, J.M., Naturwissenschaften 1975, 62, 357.
CrossRef - Flaschka, H.A., EDTA Titrations, Pergamon, Oxford, 2nd Edit., 1964.
- Gans, P., Sabatini, A and Vacca, A., Talanta 1996 , 43,1739.
CrossRef - Gans, P., Ienco, A., Peters, D., Sabatini, A. and Vacca, A., Coordination Chemistry Reviews 1999,184, 311.
CrossRef - Kadima,W. and ragenstein, D.L., J. Inorg. Biochem. 1990, 38, 227.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









