Determination of Cd(II), Zn(II) and Ag(I)in different matrixes after solid phase extraction on sodium dodecyl sulfate(SDS)-coated alumina as their 2,3 Di Hydro 2,3 paratolylQinazoline (1 H)- 4 one (DPTQO) by Flame atomic absorption spectrometric
Farveh Raoufi*1,Saideh Bagheri2,3, Pedram Nasehi2 Ebrahim Niknam4, Khodabakhsh Niknam5 and Hamid Reza Farmani6
1Chemistry Department, Islamic Azad University, Ilam Branch, Ilam, Iran 2Chemistry Department, Islamic Azad University, Dashtestan, Iran 3Young Researchers and Elite Club, Shiraz Branch, Islamic Azad University, Shiraz, Iran 4Chemistry Department, Islamic Azad University, Kangan Branch, Kangan, Iran 5Department of Chemistry, Faculty of Sciences, Persian Gulf University, Bushehr 75169, Iran 6Chemistry Department, Islamic Azad University, Ahram Branch, Ahram, Iran Corresponding Author Email: fraoufi@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320165
Article Received on :
Article Accepted on :
Article Published : 29 Feb 2016
A sensitive and selective solid phase extraction procedure for the determination of traces of Cd(II), Zn(II) and Ag(I) ions has been developed. An alumina-sodium dodecyl sulfate (SDS) coated on with 2,3 Di Hydro 2,3 paratolylQinazoline (1 H)- 4 one (DPTQO). The influences of the analytical parameters including pH and sample volume were investigated.Common coexisting ions did not interfere on the separation and determination of analytes under study. The adsorbed analytes were desorbed by using 6mL of 4 mol L−1 nitric acid. The responses are linear 0.02–0.85 µg mL-1 for Cd2+ ion0.01–0.90 µg mL-1 for Zn2+and0.02–0.92µg mL-1for Ag+ detection limit for Cd(II), Zn(II) and Ag(I) ions were found to be 1.4, 1.3 and1.12(ng mL-1), respectively.It was found that the recovery for Cd2+, Zn2+and Ag+ ions were 97.7, 98.2 and 98.0 with RSD of 1.9, 1.8 and 1.7. It was also observed that recovery for repeated recovery on the same solid phase not varies more than 3%. The presented procedurewas successfully applied for determination of analytes in radiology wastewater, amalgam, natural water and blood samples.
KEYWORDS:Surfactant coated on alumina; Solid phase extraction; Determination; recovery; Atomic absorptions pectrometry
Download this article as:| Copy the following to cite this article: Raoufi F, Bagheri S, Nasehi P, Niknam E, Niknam K, Farmani H. R. Determination of Cd(II), Zn(II) and Ag(I)in different matrixes after solid phase extraction on sodium dodecyl sulfate(SDS)-coated alumina as their 2,3 Di Hydro 2,3 paratolylQinazoline (1 H)- 4 one (DPTQO) by Flame atomic absorption spectrometric. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Raoufi F, Bagheri S, Nasehi P, Niknam E, Niknam K, Farmani H. R. Determination of Cd(II), Zn(II) and Ag(I)in different matrixes after solid phase extraction on sodium dodecyl sulfate(SDS)-coated alumina as their 2,3 Di Hydro 2,3 paratolylQinazoline (1 H)- 4 one (DPTQO) by Flame atomic absorption spectrometric. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14393 |
Introduction
Trace metals are widely spread in environment and may enter the food chain from the environment. Some trace metals are essential elements and play an important role in human metabolism. On the other hand, at higher concentrations all of the metals are recognized as potentially toxic [1, 2]. Therefore determination of trace heavy metals in different environmental samples is of great interest to analytical chemists. Recently, solid phase extraction has been successfully utilized for the preconcentration and separation of trace metal ions [3, 4]. These difficulties and limitations can be overcome with doing a preliminary separation and/or preconcentration step and subsequent measurement by FAAS to decrease the matrix interference (by its simplification) or improve in the detection limit[5,6].Various reconcentration separation methods including solid-phase extraction, membrane filtration, and electroanalytical techniques have been used for this purpose [7-10]. Solid phase extraction (SPE) composed of two distinct stages; a) selective and reversible binding of analytes to a solid support (modified); and b) their subsequent elution with a small volume of solvent that make possible simultaneous simplified matrices and enhance in sensitivity. Among various sorbents, silica gel chemically bonded with various organic compounds has received great attention due to its good mechanical and thermal stability, less susceptibility to swelling and shrinking [11-15]. The SPE can easily be adapted for FAASto improve the detection limit and selectivity of determinations. In our laboratory using various types of sorbents including activated carbon [16], SDS-coated alumina [17], modified chromosorb [18],and modified polyvinyl chloride [19] separation and/or preconcentration of metal ions has been gaining popularity because of its high concentrating ability and simple operation.Among the various solid phases, those obtained by immobilizationof chelating agent on support viz. alumina and silica gel havegained much attention with high repeatability and large lifetime.The design of a stable and selective solid phase sorbents for separation and preconcentration of a targetmetal ion depends on different factors such as the nature of solid support, its surface area and activity [20]. Among these adsorbents, alumina is an important place in the solid phase extraction studies of heavy metal ions [21]. A new modification mode for the alumina to use in solid phase extraction works was introduced by Hiraide et al. [22]. The organic reagent is incorporated in the cores of admicelles of sodium dodecyl sulfate attached to alumina surfaces. New organic reagents are immobilized on surfactant coated on alumina for separation and enrichment of different metal ions [23,24], and recently polyaromatic hydrocarbon as well [25]. The purpose of this work is to investigate the feasibility of absorption of these ions including Cd(II), Zn(II) and Ag(I) ions on SDS coated on alumina modified with 2,3 Di Hydro 2,3 paratolylQinazoline (1 H)- 4 one (DPTQO). The parameters including pH of sample, amount of ligand and solid phase, type of eluting agent and time and flow rate were optimized.
Experimental
Instruments
All the determinations of the analytes were carried out using a Sens AA GBC double beam atomic absorption spectrometer (AAS) equipped with deuterium background corrector. Hollow cathode lamps were used as radiation sources and the operational conditions of the equipment were established according to the manufacturer’s recommendations for each element. An adjustable capillary nebulizer and supplies of acetylene and air were used for the generation of aerosols and atomizations. The UV/Vis spectra were obtained from a Perkin-Elmer, model Lambda 2 spectrophotometer. A Genway model 3510 pH/Ion meter with a combined glass electrode was used for pH measurements. Laboratory glassware was kept overnight in 10% nitric acid solution. Before use, the glassware was rinsed with distilled deionized water and dried in a laminar flux cabinet.
Reagents
Doubly distilled deionized water was used throughout the experimental work. Stock solutions of all understudy elements were prepared by dissolving appropriate amount of ultrapure salt obtained from Merck (Darmstadt, Germany) in 4M HNO3. Working standard solutions were obtained by appropriate dilution ofthe stock standard solution. The pH was adjusted by addition of 0.1mol L−1 nitric acid or 0.1 mol L−1 potassium hydroxide. The Al2O3 and sodium dodecyl sulfate (SDS) were purchased fromMerck. The SDS solution was prepared by dissolving 1.0 g of surfactantin 100mL volumetric flask while stirring.The ligand 2,3 Di Hydro 2,3 paratolyl Qinazoline (1 H)- 4 one (DPTQO) was synthesized according to literature [26].
Preparation of 2,3 Di Hydro 2,3 paratolylQinazoline (1 H)- 4 one (DPTQO).
A mixture of isatoic anhydride (1 mmol), para methylbenzaldehyde (2mmol), and ammonium acetate or primary amine (1.1 mmol) in ethanol (5 mL) was added silica-bonded N-propylsulfamic acid (0.1 g) and heated at 800C in an oil bath. After completion of the reaction, as indicated by TLC, the reaction mixture was filtered and remaining washed with warm ethanol (2-5 mL). After cooling, the corresponding 2,3-dihydroquinazolinone products were obtained which purified by recrystallization from hot ethanol. The recovered catalyst was dried and reused for subsequent runs. The product was purified by column chromatography on silica gel [eluent: EtOAc/n-hexane (1:3)] to give pure 2,3 Di Hydro 2,3 paratolyl Qinazoline (1 H)- 4 one (DPTQO) in 90% yield, figure 1, was synthesized according to literature[26].
Mp: 243-245 ºC
1H NMR (DMSO-d6, 400 MHz) δ: 2.22 (s, 3H), 2.26 (s, 3H), 6.18 (d, 1H, J= 2.8 Hz), 6.68-6.75 (m, 2H), 7.09-7.15 (m, 6H), 7.23-7.28 (m, 3H), 7.55 (d, 1H, J= 2.5 Hz), 7.72 (dd, 1H, J1= 7.8 Hz, J2= 1.5 Hz).
13C NMR (DMSO-d6, 100 MHz): 20.6, 72.6, 114.7, 115.4, 117.4, 126.0, 126.4, 127.8, 128.9, 129.0, 133.6, 135.2, 137.5, 137.9, 138.3, 146.5, 162.2.
IR (KBr) (cm-1): 3265, 3078, 1640, 1614, 1516, 1475, 1383, 1250, 1152, 1112, 1010, 818, 765, 752, 51
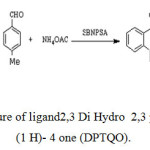 |
Figure 1: The structure of ligand2,3 Di Hydro 2,3 paratolylQinazoline (1 H)- 4 one (DPTQO). Click here to View figure |
Preparation of solid phase
A 10mL of sodium dodecyl sulfate (SDS)– DPTQO solution was added to 30mL of water solution containing 1 g of the Al2O3 particles.While shaking the suspension with a stirrer, the pH was adjusted to 2 with 2mol L−1 hydrochloric acid to form DPTQO impregnatedad micelles on alumina particles. After mixing for15 min, the supernatant solution was discarded and the remaining was used as modified solid phase. The solid phase was purifiedby passing 4mL of 4 mol L−1 HNO3and neutralized with0.1mol L−1 KOH. The sorbent in refrigerator is stable for at leastone month. The SDS concentration was fixed below the CMC of SDS(8×10−3mol L−1) to avoid formation of micelles in the aqueoussolution.
Application to Real Samples
Real samples including blood samples were treated as described previously [27]. Then the Test Procedure described above was applied. For the application of the present procedure to amalgam alloy, an accurately weighed 0.4 g portion of each amalgam alloy sample was digested as described previously [28,29]. and dissolved in the smallest volume of water, and the solution was transferred to a 100 mL volumetric flask by adjustment of the pH of the solution to the desired value; then the Test Procedure described above was carried out. The waste radiographic samples were prepared for the measurement of their cadmium, zinc and silver ions content as follows. To 20 mL sample were added about 10 mL 4M nitric acid and 10 mL water. The solution was boiled until its volume was reduced to20 mL. The resulting solution was neutralized with NaOH solution to the desired pH value and filtered. The filtrate and washings were diluted to 50 mL in a volumetric flask, and then the Test Procedure described above was applied.
Results and Discussion
The adsorption of SDS on alumina is highly dependent on the solution pH. Negatively charged SDS was more effectively andnearly quantitatively retained (about 99% even in 2mol L−1 nitricacid) on the positively charged alumina surfaces at pH 1–4. Therefore,retention of organic compounds on SDS-coated alumina[30–32] occurs. This phenomenon greatly increases by decreasing the pH due to the higher charge density on the mineral oxide surface [30,31]. The anionic surfactant SDS is effectively retained on positively charged alumina surfaces via formation of self-aggregates [32] over a wide pH range (1–6), whereas very little amount of SDS could be retained on inert surface of alumina. Therefore, alumina is essential for the preparation of surfactant-coated sorbents. It was also confirmed that, while solid phase of alumina or alumina coated with SDS show low tendency for retention of metal ions, the solid phase of immobilized Schiff base on surfactant-coated alumina was capable of retaining these metal ions from the sample solution quantitatively.Therefore, it was chosen as the sorbent for subsequent work.
Investigation of complexation of ligand with metal ions
The 2,3 Di Hydro 2,3 paratolylQinazoline (1- H)- 4 one (DPTQO),withone oxygen, two nitrogen inwater at neutral pH. It is known that the Schiff bases ligands form very stable complexes with transition metal ions. The resulting complexes have attracted increasing attention, generally due to their reactivity mainly in the area of binding small molecules. Consequently,we study the complexation of Cd2+, Zn2+and Ag+ions inmethanol. Typical spectra and moleratio plot were depicted in Figure2, for Ag+ion.As it were observed, significant apparent changes in the spectrumof ligand, on addition of metal ions indicate that, the ligandhas strong interaction with metal ions and is an efficient sorbentfor trace metal enrichment.Further analyzing the absorbance at maximum wavelength using Kinfit program the stoichiometry and stability constant of DPTQO complexes with under study metal ions.The stability constant for complexation of this ligand with thesemetal ions is as following.For ML the stability constant is 4.0±0.01 for Cd2+ ion,5.8±0.04 for Zn2+ ion and 5.8±0.01 forAg+ ion, which lead to following order Cd2+<Zn2+<Ag+ ion.He cumulative stability constant forML2 is 9.9±0.04 for Ag+ ion,7.0±0.04 for Cd2+ ion and 10.7±0.07 for Zn2+ ion. This nature seems to be not originated from their backbone structure of ligandsbut mainly from the use of ligand-N-donors on the complexation[33,34].The unique nature of Schiff base-N-donor is enhanced by the existence of widely spread conjugation system.The extraction mechanism corresponds to a cation exchange,in which a complex of stoichiometric formula (ML or/and ML2) isformed and liberating at the same time 2 mol H+ ions into solution.The results of the present investigation show that the reagent H2Lcan be successfully used for the quantitative extraction of heavymetals ions. The authors decided to use DPTQO as a suitable modifier for the selective concentration and extraction of some heavy and transition metal ions on SDS-coated alumina.
![Figure2:UV–visible spectra for titration of L (7.41×10−5mol L−1) with Ag+ (1.00×10−3mol L−1) in MeOH (T=25◦C and I = 0.05 mol L−1). (a) The molar ratio plot inλmax = 545 nm. (b) The corresponding computer-fitted curve of absorbance vs. [Ag+/L].](http://www.orientjchem.org/wp-content/uploads/2016/03/Vol32_No1_Dete_Far_Fig2-150x150.jpg) |
Figure 2: UV–visible spectra for titration of L (7.41×10−5mol L−1) with Ag+ (1.00×10−3mol L−1) in MeOH (T=25◦C and I = 0.05 mol L−1). (a) The molar ratio plot inλmax = 545 nm. (b) The corresponding computer-fitted curve of absorbance vs. [Ag+/L]. Click here to View figure |
Effect of pH on metal ion recovery
pH is one of the important factors which affect the efficiency of retention/elution metal ions by solid phase extraction [35–37]. The effect of pH was studied by varying pH in the range of 5.5–12. It can be seen from Figure 3. that the adsorption amounts of mixed hemimicelles systems decreased obviously with the increasing of pH. Especially, when pH was above the potential of zero charge (PZC) of alumina (about 10.0), the adsorption amount of SDS-coated alumina had a sharp decrease. With increasing the pH, the positive charge of alumina surface reduced gradually and became negative charged when the pH was above its PZC. As a result, the SDS molecules desorbed from alumina surface gradually and begin to decrease. On the other hand the progressive decrease in the retention of these metal ions at a low pH is due to the competition of the hydrogen ion with the metal ions for complexation and binding to Schiff base. The decrease in absorption at pH >9.5 is probably due to the precipitation of Cd2+, Zn2+and Ag+ions as insoluble M(OH)2 or M(OH)+. To achieve high efficiency and good selectivity, a pH of 9.5 was selected for subsequent work.
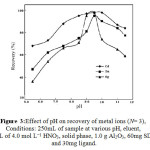 |
Figure 3: Effect of pH on recovery of metal ions (N= 3), Conditions: 250mL of sample at various pH, eluent, 8mL of 4.0 mol L−1 HNO3, solid phase, 1.0 g Al2O3, 60mg SDS and 30mg ligand. Click here to View figure |
Effect of amount of ligand on metal ion recovery
In order to investigate the optimum amount of ligand for the quantitative extraction of the metal ions by the SDS-coated alumina, the extraction was conducted by varying the amount of ligand from 0 to 60mg. Therefore, solid phase comprises of various amount of ligand coated on constant amount of alumina were prepared and enrichment experiment was carried out, results are shown in Figure 4. As can be seen, with increasing amount of ligand up to 30mg an increase in recoveries can be achieved and further increase does not enhance the efficiency. The various amount of solid phase has been used for the preconcentration of understudy Cd2+, Zn2+ and Ag+ ions and results display that up to 1.0 g of solid phase, the extractions efficiency was increase and further addition has not significant effect on recoveries.
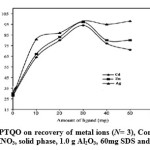 |
Figure 4: Effect of amount of DPTQO on recovery of metal ions (N= 3), Conditions: 250mL of sample at pH 9.5, eluent, 8mL of 4.0 mol L−1 HNO3, solid phase, 1.0 g Al2O3, 60mg SDS and different amount of ligand. Click here to View figure |
Amount of SDS
The influence of SDS amount on the percentage of complexed ions ad-solubilized was investigated by shaking 250mL of a solution containing 50µg of each Cd2+, Zn2+and Ag+ions with solid phase containing various amounts of SDS, 1 g γ- Al2O3 and 10 mg DPTQO at pH 9.5 for 90min with stirring rate 300 rpm. In the absence of SDS, metal ions retained on DPTQOalumina with 60% lower efficiency for all ions. The retention of metal ions on hemi-micelles, which have a hydrophobic surface, was clearly dependent on analyte complex polarity. Therefore, addition of SDS is necessary. The formation of minute amounts of ad-micelles was essential to achieve complete ad-solubilization of chelates of these ions. At surfactant concentrations higher than about 60 mg/g alumina, a decrease in the retention percentage of ions was observed, as a result of the formation of micelles (Figure5). The adsorption amount began to decrease when the SDS added exceeded 60mg for 1.0 g of alumina. It can be explained by the fact that with more SDS added, its molecules began to form micelles in the bulk aqueous solution; moreover, the micelles make the DPTQO distribute into the bulk solution again. In this region the slope of alumina adsorption curve is flat, which indicated that the interactions between DPTQO and SDS-coated alumina were strong. As a result, it was more difficult for SDS-coated alumina to desorb DPTQO into aqueous solution.
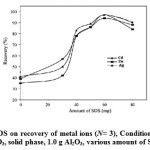 |
Figure 5: Effect of amount of SDS on recovery of metal ions (N= 3), Conditions: 250mL of sample at PH=9.5, eluent, 6mL of 4.0 mol L−1 HNO3, solid phase, 1.0 g Al2O3, various amount of SDS and 30mg ligand. Click here to View figure |
Eluent types and eluent volume
In order to choose the most effective eluent for desorbing the Cd2+,Zn2+and Ag+ ions from the sorbent surface, a series of eluents (different acids) were used in order to find a selective eluent for desorbing enriched ions from sorbent surfaces. A total of 6ml of 4.0 M of the above-mentioned eluents were used for desorbing the adsorbed ions. The results (Table 1) showed that the recovery is best when nitric acid solution was used as the eluent. The influence of the concentration of nitric acid on the desorption of these ions was studied. For desorbing 40 µg of ions, already adsorbed on 1.0 g of adsorbent, different concentrations of the eluent (nitric acid) have been used. At concentrations more than 4 M the extraction efficiency reached maximum.
Table 1:.Effect of type and concentration of eluting agent on recovery of analytes.
|
Eluent |
Recovery (%) |
||
|
Cd |
Zn |
Ag |
|
|
8mL 4mol L−1 H3PO4 |
28.7 |
31.8 |
26.4 |
|
8mL 4mol L−1 H2SO4 |
12.4 |
16.2 |
14.5 |
|
8mL 4mol L−1 HCL |
53.6 |
58.6 |
54.2 |
|
8mL 4mol L−1 CH3COOH |
29.6 |
32.4 |
30.1 |
|
8mL 2mol L−1 HNO3 |
64.5 |
68.0 |
66.7 |
|
8mL 3mol L−1 HNO3 |
70.6 |
73.2 |
71.2 |
|
8mL 4mol L−1 HNO3 |
98.0 |
98.5 |
98.1 |
|
8mL 5mol L−1 HNO3 |
84.0 |
86.1 |
81.2 |
|
4mL 4mol L−1 HNO3 |
90.0 |
92.1 |
89.2 |
|
6mL 4mol L−1 HNO3 |
98.0 |
98.5 |
98.1 |
|
9mL 4mol L−1 HNO3 |
98.0 |
98.5 |
98.1 |
|
10mL 4mol L−1 HNO3 |
98.0 |
98.5 |
98.1 |
Effect of time and rate of shaking
The efficiencies of the analyte deposition depend on the contact time and stirring rate of sample with the solid phase. It is necessary to require the preconcentrate of metal ions in short time. In this regard, a replicate six set of analyte and adsorbents were prepared and stirring at different time intervals (20–120 min) with stirring rate of 100–400 rpm. It was observed that 90 minmixing at 300rpm stirring rate to optimum recoveries of Cd2+, Zn2+ and Ag+ ions. On the other hand, a set of similar solid phase extraction were carried out at 300rpm while keeping the temperature at 25–60◦C for 90min. Later on, the residual concentration was determined where the metal ion uptake was estimated. The DPTQO adsorption amounts of SDS-coated alumina systems decreased with increasing temperature. The decreased adsorption amounts suggested that the two-adsorption systems were exothermic. With the increasing of temperature the adsorbate–adsorbent bonds were weakened and DPTQO had desorption trend from the solid phase to solution phase. It can also be proved by the changes of SDS adsorption amounts. With the temperature increasing, the bond between alumina and SDS became weak and partial SDS molecules began to desorb from alumina surface.
Investigation of method performances
Added 250 mL of solution of metal ions in the range of 0.01–1.00 gmL−1 with coated adsorbents at the optimum conditions, to obtain the calibration curves, repeatability and reproducibility. The responses are linear 0.02–0.85µg mL-1 for Cd2+ ion, 0.01–0.90 µg mL-1for Zn2+ ion, 0.02–0.92 µg mL-1 for Ag+ ion. The sensitivity of developed method was reproduced by detecting the limit of detection (LOD), defined as the lowest concentration of metal ions below which quantitative sorption of the metal ion by basic alumina is not perceptibly seen. The limits of detection (LOD) of the present work were calculated under optimal experimental conditions after application of the preconcentration procedure to blank solutions (without samples or standards). The limits of detections based on three times the standard deviations of the blank (N= 5, LOD= Xb+3s, where LOD is the limits of detection and Xb is the blank value) for Cd2+, Zn2+and Ag+ions to be 1.4, 1.3 and1.2 (ng mL-1), respectively. were found that the recovery for Cd2+, Zn2+and Ag+ ions were 97.7, 98.2, 98.0 and 95.0 with RSD of 1.9, 1.8 and 1.7.( Table 2). It was also observed that recovery for repeated recovery on the same solid phase not varies more than 3%. Therefore, understudy solid phase can be repeatedly use without considerable loss of uptake capacity[38].
Table 2: Specification of Method at Optimum Conditions for Each element According .
|
parameters |
Value for each ions |
||
|
Cd |
Zn |
Ag |
|
|
Linear Range (µg mL-1) |
0.02-0.85 |
0.01-0.90 |
0.02-0.92 |
|
Detection Limit (ng mL-1) |
1.4 |
1.3 |
1.2 |
|
Loading Capacity (mg g-1) |
0.45 |
0.46 |
0.61 |
|
RSD % |
1.9 |
1.8 |
1.7 |
|
Recovery % |
97.7 |
98.2 |
98.0 |
Effect of foreign ions
To assess the possible applications of the procedure, the effect of foreign ions or/and often accompany analyte ions in various real samples, which are interfere with the determination of Cd2+, Zn2+ and Ag+ ions, was examined at optimized conditions. The results are given in Table 3. A fixed amount of understudy analytes were taken with different amounts of foreign ions and proposed procedure was followed. The recoveries of analytes were higher than 95%. Tolerable limit was defined as the highest amount of foreign ions that produced an error which is not exceeding 5%, in the determination of investigated analyte ions by the combination of the SDS-coated alumina solid phase extraction procedure followed by the flame atomic absorption spectrometry. It was observed that, most of the ion tested, have no significant effect on the recoveries of analyte ion. Cd2+, Zn2+ and Ag+ ions were quantitatively recovered in the presence of optimum mount of alkaline, earth alkaline, transition and heavy metal ions as well as some anions.
Table 3: Effects of the matrix ions on the recoveries of the examined metal ions (N=3).
|
Ion |
Added As |
Tolerance Limit Ion mg mL-1 |
||
|
Cd |
Zn |
Ag |
||
|
Na+ |
NaCl, |
3000 |
5000 |
3000 |
|
K+ |
KCl |
2500 |
2500 |
2500 |
|
Ca2+ |
CaCl2 |
2500 |
2500 |
2500 |
|
Cl- |
NaCl |
2500 |
2500 |
2500 |
|
SO42- |
(NH4)2SO4 |
2500 |
2500 |
2500 |
|
Mg2+ |
MgCl2 |
750 |
1000 |
800 |
|
HCO3– |
NaHCO3 |
800 |
1000 |
800 |
|
PO43- |
Na3PO4 |
2500 |
2500 |
2500 |
|
Cu2+, Co2+, Hg2+, Ni2+, Al3+, Cr3+,Ba2+, |
Nitrate salt |
500 |
600 |
500 |
Real samples – evaluation of method
The proposed method was applied for the determination of Cd2+, Zn2+ and Ag+ ions by the standard addition technique in radiology wastewater, amalgam, natural water and blood samples. including well radiology wastewater and amalgam samples (Table 4). The results of in natural water and blood samples.as mentioned in the Experimental section are summarized in (Table 5). Notably, the determination of Cd2+, Zn2+ and Ag+ ions in radiology wastewater, amalgam, natural water and blood samples. is remarkably accurate by the proposed method. The results of the determination of Cd2+, Zn2+ and Ag+ ions in the in radiology wastewater, amalgam, natural water and blood samples. as mentioned in the experimental section are listed in Table 5.
Comparison with literature
A comparison of the proposed system with other preconcentration procedures using several sorbents is given in Table 6 [39–42], the proposed method shows comparable capacity level, lower detection limit, and wider linear range and is a convenient, safe, simple, rapid and inexpensive method for the determination of trace quantities of these cations in radiology wastewater, amalgam, natural water and blood samples with satisfactory results. The proposed preconcentration system shows goodpreconcentration factors with reasonable preconcentration time over other preconcentration methods [43–45]. As seen from Table 6, the detection limit for the proposed method was comparable to those given by the many methods.
Table 4: Recovery of trace elements from radiology wastewater and amalgam samples after application of presented flotation procedure(N=3)
|
Ion |
Added, µg L-1 | Found, µg L-1 | RSD % | Recovery % | ||
|
Radiology wastewater |
||||||
|
Cd |
0 0.5 |
0.17 0.66 |
1.6 1.2 |
— 98.4 |
||
|
Zn |
0 0.5 |
6.6 8.6 |
1.5 1.0 |
— 102.3 |
||
|
Ag |
0 0.5 |
1.6 2.1 |
1.4 1.0 |
— 101.1 |
||
| Amalgam | ||||||
|
Cd |
0 0.5 |
0.06 0.51 |
1.4 1.1 |
— 103.6 |
||
|
Zn |
0 0.5 |
1.3 1.9 |
1.3 1.2 |
— 101.6 |
||
|
Ag |
0 0.5 |
0.4 0.9 |
1.2 0.9 |
— 102.1 |
||
Table 5: Recovery of trace elements from natural water and blood samples after application of presented flotation procedure(N=3)
| Ion | Added, µg L-1 | Found, µg L-1 | RSD % | Recovery % | |||
| Blood | |||||||
|
Cd |
0 10 |
0.7 1.8 |
1.5 1.2 |
— 103.0 |
|||
|
Zn |
0 10 |
2.0 3.5 |
1.4 1.0 |
— 103.2 |
|||
|
Ag |
0 10 |
0.02 013 |
1.2 0.9 |
— 100.6 |
|||
| Natural water | |||||||
|
Cd |
0 10 |
0.5 1.4 |
1.2 1.1 |
— 101.2 |
|||
|
Zn |
0 10 |
3.4 6.8 |
1.4 1.2 |
— 100.3 |
|||
|
Ag |
0 10 |
0.04 0.07 |
1.5 1.2 |
— 99.0 |
|||
Table 6: Comparative data from recent papers on preconcentration studies a.
|
ytes |
Methodand apparatus |
EF |
DL (μg/L) |
R.S.D. (%) |
5 |
|
Cu, Cd, Pb, Zn, Ni, Co |
SPE/FAAS |
80.0 |
0.16–0.60 |
1–17 |
[39] |
|
Cd(II), Pb(II) |
SPE/FAAS |
288.0 |
0.07–0.09 |
1.2 – 2.1 |
[40] |
|
Cd(II) |
SPE/FAAS |
75.0 |
0.6 |
<3.7 |
[41] |
|
Cd(II), Zn(II), Ni(II) |
SPE/FAAS |
80.0 |
2.1–2.3 |
1.23–1.31 |
[42] |
|
Cd(II), Zn(II), Ag(I) |
SPE/FAAS |
80.0 |
1.4, 1.3, 1.2 |
<3.6 |
Present work |
aEF: enrichment factor,DL: detection limit,R.S.D.:relative standard deviation; SPE: solid phaseextraction.
Conclusion
The DPTQO loaded on SDS-coated alumina is sensitive and accurate adsorbent for determination of trace amounts of Cd2+, Zn2+ and Ag+ ions. The results presented in this paper have confirmed the applicability of the separation and preconcentration of metals. This method is simple and there is no necessity for elaborating a cleanup procedure, but the retained metals were simply eluted with 6mL of 4 mol L−1 HNO3 and were analyzed by FAAS. Each solid phase can be usedfor at least 10 successive analyses without considerable change in Cd2+, Zn2+ and Ag+ ions recovery. As a result it can be concluded that the proposed procedure can be satisfactorily considered as an alternative application for preconcentration and separation of traces of Cd2+, Zn2+ and Ag+ ions from aqueous solute. The performance characteristics of the proposed method and other selected SPE/FAAS systems for comparative purposes in the literature are given in Table 5. The proposed procedure shows good detection limits and precision with reasonable eluent volume, flow rate and preconcentration factor over other SPE off-line preconcentration methods [46–51]. The method is simple, accurate can be applied for the determination of Cd2+, Zn2+ and Ag+ ions inradiology wastewater, amalg am, natural water and blood samples[52].
Acknowledgment
The authors express their appreciation to the Graduate School and Research Council of the Ilam Azad University for the financial support of this work.
References
- S. Baytak, A. RehberTurker;Talanta,2005,65, 938.
CrossRef - G. Khayatian, S. Pouzesh;J. Iran.Chem. Soc.,2007,4, 490.
CrossRef - M. Ghaedi, M. Montazerozohori, E. Nazari, R. Nejabat;Human & Experimental Toxicology. 2013,32, 687.
CrossRef - N. Pourreza, J. Zolgharnein, A. R. Kiasat, T. Dastyar;Talanta,2010, 81, 773.
CrossRef - K. Mortazavi, M. Ghaedi, M. Montazerozohori, M. Roosta; Fresenius Environ. Bull,2011, 20, 2847.
- M. Ghaedi, K. Niknam, K. Taheri, H. Hossainian, M. Soylak; Food Chem. Toxicol,2010,48, 891.
CrossRef - A. Lopez-Gonzalvez, M.A. Ruiz, C.Barbas; J. Pharm. Biomed.Anal,2008,48, 340.
CrossRef - N. Rajesh, S.Manikandan;Spectrochim.Acta,2008,70, 754.
CrossRef - V.A.Lemos, L.S.G. Teixeira, M.A.Bezerra, A.C.S. Costa, J.T. Castro, L.A.M. Cardoso, D.S. de Jesus, E.S. Santos, P.X.Baliza, L.N. Santos; Appl. Spectrosc. Rev,2008, 43, 303.
CrossRef - K.Farhadi, N.Abdollahnezhad, R.Maleki; Int. J. Environ. Anal.Chem, 2008, 88, 725.
CrossRef - M. Tuzen, E. Sesli, M. Soylak; Food Control,2007,18, 806.
CrossRef - Y.M. Cui, X.J. Chang, X.B. Zhu, H.X. Luo, Z. Hu, X.J. Zou, Q. He;Microchem. J,2007,87, 20.
CrossRef - K. Mortazavi, M. Ghaedi, M. Roosta, M. Montazerozohori; Indian J. Sci. Technol, 2012, 5, 1893.
- T.G. Kazi, N. Jalbani, M.K. Jamali, M.B. Arain, H.I. Afridi, A. Kandhro, Z. Pirzado; Renal Failure. 2008,30, 737
CrossRef - M. Ghaedi, E. Niknam, A. Shokrollahi, E. Niknam, H.R. Rajabi, M. Soylak; J. Hazard. Mater,2008,155,121.
CrossRef - M, Ghaedi, A, Shokrollahi, K, Niknam, E, Niknam; Journal Chinese Chemical Society, 2009,56, 150.
CrossRef - M. Ghaedi, H. Tavallali, M. Montazerozohori, E. Zahedi, M. Amirineko, S. Khodadoust, G. Karimipour; Environ. Monit. Assess, 2012,184, 6583.
CrossRef - N. Pourreza, K. Ghanemi; J. Hazard.Mater,2010,178, 566.
CrossRef - M. Ghaedi,E. Niknam; Bull. Chem. Soc. Ethiop,2010, 24(1), 1.
CrossRef - M. Ghaedi, E. Asadpour, A. Vafaie; Bull.Chem. Soc. Jpn,2006,79,432.
CrossRef - R.P. Budhiraja; New Age International Publishers, NewDelhi, 2004.
- V. Smuleac, D.A. Butterfield, S.K. Sikdar, R.S. Varma, D. Bhattacharyya; J. Membr.Sci,2005,251, 169.
CrossRef - E.M. Soliman, M.B. Saleh, S.A. Ahmed;Talanta,2006,69, 55.
CrossRef - S. Dadfarnia, A.M.H. Shabani, M. Gohari;Talanta,2004,64, 682.
CrossRef - T. Saitoh, S. Matsushima, M. Hiraide; J. Chromatogr.A,2005,1069, 271.
CrossRef - K. Niknam, N.Jafarpour, E.Niknam; Chinese Chemical Letters,2011,22, 69.
CrossRef - A.Shokrollahi, H.E.Haghighi, E.Niknam, K.Niknam;Quím. Nova São Paul,2013,36,3.
- M.Ghaedi, M.R.Fathi, A.Shokrollahi, F.Shajarat;Anal.Lett,2006,39,1171.
CrossRef - F. Ahmadi, E. Niknam, K. Niknam, and A.Khanmohammadi;Arabian Journal for Science and Engineering,2011,36, 47.
CrossRef - F. Merino, S. Rubio, D. Perez-Bendito; Anal.Chem,2003,75,6799.M. Cantero, S. Rubio, D. Perez-Bendito; J. Chromatogr.A,2005,1067,161.
- J. Li, Y. Shi, Y. Cai, S. Mou, G. Jiang; Chem. Eng. J.,2008,140,214.
CrossRef - M. Ghaedi, J. Tashkhourian, A. Pebdani, A. Batol, F. Nami; Korean Journal of Chemical Engineering., 2011,28, 2255.
CrossRef - H. Tavallali, A.M. Attaran; International Journal of Chem Tech.,2010, (3) ,1724.
- M. Soylak, L. Elc,M. Dogan; Anal.Lett.,1997,30, 623.
CrossRef - M.V. Taseska, I.B. Karadjova, T.Z. Stafilov; Eurasian J. Anal.Chem.,2008,3, 1.
- P. Liang, E. Zhao, Q. Ding, D. Du; Spectrochim.Acta Part.B.,2008,63,714.
CrossRef - IUPAC, Nomenclature; Pure Appl. Chem.,1976, 45,105.
CrossRef - M. Tuzen, M. Soylak, L. Elci; Anal.Chim.Acta.,2005,548, 101.
CrossRef - F. Marahel, M. Ghaedi, A. Shokrollahi, M. Montazerozohori, S. Davoodi;Chemosphere.,2009,74, 583.
CrossRef - P. Liang, and L. Peng;Talanta.,2010, 81, 673.
CrossRef - M. Ghaedi, K. Niknam, S. Zamani, H.AbasiLarki , M. MostafaRoosta, M. Soylak; Materials Science and Engineering.,2013,3,3180.
CrossRef - M. Soylak, I. Murat; Food Anal.Methods.,2012,5,1003.
CrossRef - L. Elci, A.A. Kartal, M. Soylak; J. Hazard.Mater.,2008,153,454.
CrossRef - M. Soylak, E. Yilmaz; J. Hazard.Mater.,2010,182,704.
CrossRef - M. Soylak, L. Elc,M. Dogan; Bull.,1996,5,148.
- B. Mandal, U.S. Roy; Indian J. Chem. A.,2008, 47, 1497.
- T. Shamspur, T.A. Mostafavi, I. Sheikhshoaie; J. AOAC Int.,2008,91, 865.
- B.W. Yang, Z.F. Fan, Spectrosc.,2008,29, 193.
- E. Birinci, M. Gulfen, A.O. Aydın; Hydrometallurgy.,2009,95, 15.
CrossRef - M.E. Mahmoud, A.A. Yakout, S.B. Ahmed, M.M. Osman; J. Liquid Chromatogr. Related Technol.,2008,31, 2475.
CrossRef - D.H. Chen, M. He, C.Z. Huang, B. Hu; Atom.Spectrosc.,2008,29, 165.

This work is licensed under a Creative Commons Attribution 4.0 International License.









